Abstract
Background Anemia of inflammation (AI) is an immune-driven disorder that displays reduced erythropoietic activity and dysregulation of iron homeostasis. It is associated with a number of underlying diseases including myelofibrosis (MF) and chronic kidney disease (CKD) and caused by inflammatory cytokines which increase hepcidin levels. Increased hepcidin leads to sequestered iron in macrophages, decreased iron absorption and decreased serum iron. Interfering with HJV function and suppressing the production of hepcidin results in increases in serum iron in healthy volunteers has been observed with another humanized monoclonal antibody (mAb, DISC-0974). PK/PD results from a Phase 1 clinical trial with DISC-0974 in healthy volunteers support monthly subcutaneous (SC) dosing.
DISC-0998 is a potent and highly selective HJV mAb with an engineered Q and L mutation (QL-mutation) in the Fc region, aimed to alter binding to the FcRn receptor, resulting in an increased PK half-life. Preclinical studies have demonstrated that DISC--0998 has biological activity, low immunogenicity potential and desirable pharmacokinetic (PK) and pharmacodynamic (PD) properties.
Objectives To evaluate the PK/PD relationships of DISC-0998 with hepcidin, serum iron, and TSAT in male cynomolgus monkeys to inform drug development strategy.
Methods Male cynomolgus monkeys were randomized to different dose groups and received a single SC (0.1, 0.3, 1.0, and 3.0 mg/kg) or intravenous (IV, 6 mg/kg) dose of DISC-0998. Blood samples were obtained at predefined time points for PK, hepcidin-25, serum iron, and total iron binding capacity (TIBC) measurements. Concentration of serum hepcidin-25 was analyzed using a qualified LC-MS/MS method. Concentrations of serum iron and TIBC were analyzed using a HITACHI7180 Chemistry Analyzer. Serum DISC-0998 concentrations were analyzed using a qualified MSD method. For comparison purposes, the results from DISC-0974 monkey PK/PD studies following 0.3, 1, and 6 mg/kg SC were also included. WinNonlin (PhoenixTM, v8.1) was used for PK/PD calculations.
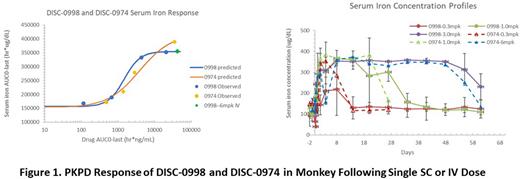
Results Following a single SC or IV dose, DISC-0998 exhibited low clearance (SC CL/F 0.14 - 0.83 mL/hr/kg; IV CL 0.14 mL/hr/kg), small volume of distribution (Vz 50 -104 mL/kg), and nonlinear PK typical for a mAb. Compared to DISC-0974, DISC-0998 exhibited lower clearance (~30 -40%), higher volume of distribution (30 - 70%), and a longer half-life (~ 2 times), which translated to longer duration of downstream PD effects. Dose-dependent hepcidin-25 reduction, along with elevations in serum iron and TSAT were also demonstrated. Based on PK and serum iron response, DISC-0998 has similar or slightly better potency in monkey compared to DISC-0974 (EAUC50 of 1495 hr*ug/mL vs. 3918 hr*g/mL, respectively).
Conclusion DISC-0998 demonstrated a favorable PK/PD profile in monkeys, allowing prediction of its PKPD profiles in humans. These data support further development of DISC-0998 for anemia of inflammation related disorders, with the potential of long intervals in between dosing.
Disclosures
Chen:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Savage:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Panwar:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Rodriguez:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Wu:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Quisel:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Yang:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal