Abstract
Introduction: The introduction of tyrosine kinase inhibitors (TKIs) has revolutionized the treatment of chronic myeloid leukemia (CML), bringing the life expectancy of patients close to that of the general population. However, TKI treatment is associated with adverse events, some of them serious or affecting quality of life. In addition, drug costs have a high economic impact, especially in low-income countries. Treatment-free remission (TFR) is an emerging goal for CML patients and it has been associated with cost savings in different country analyses.
Main Objective: Assess the economic impact of TKI discontinuation in patients included in the AST2018 Trial. Secondary objective: Estimate the long-term saving in patients included in the AST2018 Trial, considering Argentinian life expectancy.
Methods: AST2018 is a multicentric, prospective trial that recruited aged >18 years CML chronic phase patients, with confirmed typical BCR-ABL1 transcripts b3a2 and/or b2a2, under TKI treatment for at least ≥ 4 years and sustained MR4.0 for ≥ 2 years. Molecular tests were centralized in 2 harmonized laboratories and performed monthly for the first 6 months, every 2 months until the 1st year, and every 3 months until month 24. TKI was restarted at confirmed loss of major molecular response (MMR).
The economic impact was evaluated by an Excel-based budget impact model, in order to estimate the costs of the patients in first-line pharmacological treatment for CML. We considered the costs of TKI-treatment, PCRs performing and medical visits as money flow. We calculated the present value of savings, in US dollars, in patients who discontinued treatment during the trial.
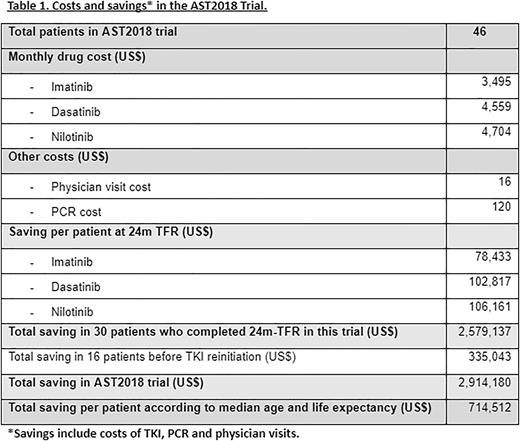
Results: Between February 2018 and March 2022, we evaluated 50 CML patients in the AST2018 trial, 46 were enrolled from 7 centers in Argentina, and were included in this economic analysis. The median patients’ age was 57.5 years (24-85). TKI treatment before discontinuation was Imatinib 34/46 (74%), Nilotinib 4/46 (9%) and Dasatinib 8/46 (17%). After 24 months since first patient was enrolled, 30/46 (65%) patients continued free from TKI, Imatinib 21/34, Nilotinib 2/4 and Dasatinib 7/8, with a median follow-up of 31.3 months (range 25.9 - 38.1). Treatment was restarted after losing MMR in 16/46 (35%) patients.
We considered the costs of TKI treatment, PCR performing and physician visits, and we calculated the estimated cost saving per patient at 24 months on TFR: Imatinib US$ 78,433; Dasatinib US$ 102,817 and Nilotinib US $106,161. For the 30 patients who persisted in TFR for 24 months, total cost saving was US$ 2,579,137. For 17 patients who lost MMR, US$ 335,043 dollars were saved previously to TKI reinitiation. Adding up both amounts, the total economic saving in AST2018 was US$ 2,914,181.
We performed an analysis of the potential cost savings of remaining in TFR beyond 24 months and according to life expectancy in Argentina (76 years), median age of patients in this trial and cost per patient/year on TKI treatment, the estimated cost saving per patient was US$ 714,512.
Conclusions: This is the first economic analysis of the AST2018 trial on TKI discontinuation in CML patients with sustained deep molecular response in Argentina. Our analysis shows that, in spite of the PCR spending, there is a considerable saving in patients on TFR. A careful approach for patients participating in TFR trials or programs will decrease the risk of relapse and make TFR a reality in our region. Potential advantages of TFR will not only impact in patients’ quality of life but also show considerable reduction in the use of economic resources. In developing countries, where lack of molecular monitoring is still an issue outside academic centers, these savings could be redirected to this unmet need making TFR feasible and a goal for the patient and the healthcare system.
Disclosures
Moiraghi:Takeda: Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Speakers Bureau. Pavlovsky:Janssen: Other: Conference; AbbVie: Other: Conference; AstraZeneca: Other; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Raffo: Membership on an entity's Board of Directors or advisory committees. Pavlovsky:Novartis, Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis, Pfizer, BMS, Pint Pharma: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal