Abstract
Cyclin-dependent kinase 9 (CDK9) is a master regulator of transcription that modulates paused RNA polymerase II (RNAPII) release through phosphorylation of its carboxy-terminal domain, resulting in productive transcription elongation and termination. CDK9 has been extensively studied as a potential target for cancer therapy in "transcriptionally addicted" hematological malignancies and solid tumors. PRT2527 is a novel, low nanomolar potent, and highly selective CDK9 inhibitor.
In a panel of hematological cancer cell lines representing B-ALL and T-ALL, PRT2527 treatment resulted in potent, concentration-dependent inhibition of proliferation and induction of apoptosis with IC50 values <100 nM. Based on these data, studies were conducted to explore the anti-leukemic activity of PRT2527 in a panel of primary adult B-ALL (N=8, including two samples with KMT2A rearrangements (MLL-r)), T-ALL (N=6), and CLL (N=10) patient samples derived from leukapheresis and maintained ex vivo in short-term cultures in growth factor and cytokine-enriched growth media. The concentration-related effects of PRT2527 treatment on primary cancer cell viability was evaluated in a plate-based luminescence assay (CellTiter-Glo®) after 48 hours of treatment. Doxorubicin, Ibrutinib and Venetoclax served as positive control therapeutic agents to assess the responsiveness of primary cultures ex vivo. Immunophenotyping, cytogenetics, and whole exome sequencing (WES) data were available for most primary samples evaluated for their responsiveness to PRT2527.
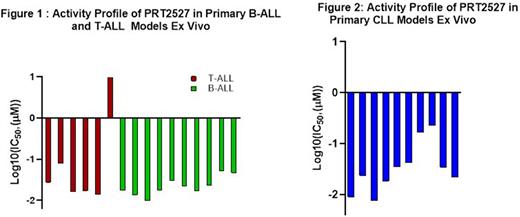
Consistent with recent observations demonstrating CDK9 inhibition in leukemias (McCalmont, 2020), PRT2527 demonstrated potent and concentration-dependent anti-leukemic activity in 10/10 B-ALL and 5/6 T-ALL primary cultures ex vivo with IC50 values <50 nM (Figure 1), independent of immunophenotype, cytogenetic profile and refractoriness to prior standard of care (SoC) therapies. Of note, 5 specific genes were highly amplified in all 10 responsive B-ALL samples - ZNF280A, PRAME, OR2T10, GGTLC2, and IGLL5, suggesting a potential common genomic "signature" of sensitivity to CDK9 inhibition. Similarly, PRT2527 treatment resulted in a potent anti-leukemic response in the panel of 10 primary CLL cultures ex vivo (Figure 2), with 8/10 models having IC50 values <40 nM, and the remaining two less responsive models having IC50 values < 225 nM. These responses were independent of the cytogenetic and immunophenotypic profiles of these CLL samples. Notably, the two less responsive models were from CLL patients who responded to Rituximab/Ibrutinib SoC therapy without further disease progression.
Confirmatory in vivo studies were conducted with two responsive primary B-ALL models - one with a KMT2A-EPS15 rearrangement and a second lacking a KMT2A rearrangement. Sublethally irradiated female NCG mice were implanted with human B-ALL cells into the lateral tail vein and cell engraftment in bone marrow (BM) and peripheral blood (PB) was assessed using huCD45/muCD45/huCD19 immunophenotyping. Following successful engraftment (> 5% huCD45+ cells in PB and BMs), PRT2527 was administered (20 mg/kg iv q2h QW) and survival and immunophenotyping analyses conducted. PRT2527 administration resulted in a significant (p<0.01) improvement in median survival in the aggressive KMT2A-rearranged primary systemic B-ALL model. Additionally, in the slower growing, indolent primary B-ALL model lacking KMT2A-rearrangement, PRT2527 administration caused a reduction in the percentage of hCD45+ B-ALL cells in the PB and BM at 6 and 10 weeks of treatment and improved body weight gain relative to control mice.
In summary, PRT2527 treatment resulted in marked and highly potent anti-leukemic activity in the panels of primary B-ALL, T-ALL, and CLL models evaluated ex vivo, and confirmation of anti-leukemic efficacy of PRT2527 in vivo was demonstrated in two systemic models of B-ALL. These data and those reported previously support the advancement of PRT2527 into clinical studies in leukemia patients, especially in patients refractory or progressing on standard therapies. PRT2527 is currently in phase 1 studies in solid tumor patients.
Disclosures
Bhagwat:Prelude Therapeutics: Current Employment. Ruggeri:Prelude Therapeutics: Consultancy, Ended employment in the past 24 months. Zhang:Prelude Therapeutics: Ended employment in the past 24 months. Mosesson:Champions Oncology: Current Employment. Killick-Cole:Champions Oncology: Current Employment. Jagannathan:Champions Oncology: Current Employment. Scherle:Prelude Therapeutics: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal