Abstract
Background: TQB3602 is a novel oral proteasome inhibitor with an acridine ring in addition to the main structure of ixazomib, which maintains better metabolic stability and higher activity and selectivity in preclinical models of multiple myeloma. TQB3602 consists of the L-malate ester with active boronic acid compound WXFL10410333 selectively targeting 20S proteasome enzyme. This is the first-in-human study to evaluate the safety, tolerability and pharmacokinetics of TQB3602 and assess the efficacy of TQB3602 combined with immunomodulators/dexamethasone regimen in relapsed/refractory multiple myeloma (RRMM).
Study Design and Methods: This study is a multi-center open-label Phase I study in RRMM, consisting of the dose escalation phase and dose expansion phase. The inclusion criteria mainly include: 1) 18 to 75 years old male or female, 2) ECOG score of 0 to 2, 3) Life expectancy ≥12 weeks, 4) RRMM had received at least two treatment regimens (including lenalidomide or thalidomide, bortezomib, and glucocorticoid). The exclusion criteria mainly include: 1) grade ≥ 2 peripheral nerve diseases, 2) grade > 1 diarrhea during the screening period, 3) ixazomib has been administered less than five half-lives before the first dose of TQB3602, 4) previous allogeneic hematopoietic stem cell transplantation. TQB3602 was administered alone in 3+3 dose escalation (at a starting dose of 0.5mg on days 1, 8 and 15 in a 28-day cycle) until toxicity or disease progression in the dose escalation phase. According to prior lines of therapy, patients with RRMM were divided into two dose expansion cohorts, including TQB3602/lenalidomide/dexamethasone and TQB306/pomalidomide/dexamethasone (TQB3602 6mg on days 1, 8, 15, lenalidomide 25mg daily or pomalidomide 4mg daily on days 1 to 21, dexamethasone 20mg on days 1-2, 8-9, 15-16, 22-23 in a 28-day cycle).
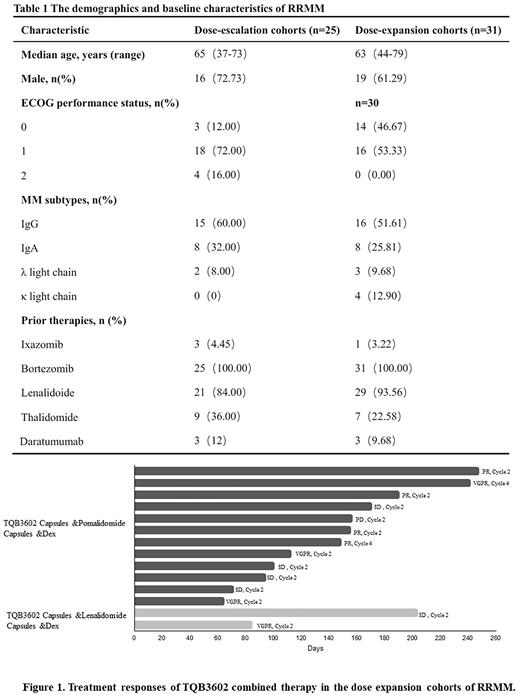
Results: The study enrolled 56 patients with RRMM including 25 patients in the dose escalation and 31 patients in the dose expansion between May 28, 2020 and July 20, 2022. The demographics and baseline characteristics were shown in table 1. Patients received single-agent TQB 3602 0.5mg to 7 mg on days 1, 8 and 15 of a 28-day cycle and two dose-limiting toxicities occurred at 7 mg, including diarrhea and grade 2 peripheral neuropathy. The maximum tolerated dose of TQB3602 was determined as 6.0mg. The terminal half-life was 23 to 152 hours after multiple dosing and the mean half-life was approximately 82 hours for 6mg TQB3602. The dose expansion cohorts recruited 29 RRMM patients receiving TQB3602/pomalidomide/dexamethasone regimen and two RRMM patients receiving TQB3602/lenalidomide/dexamethasone regimen. Among 14 response-evaluable patients in the dose expansion cohorts, eight RRMM (57%) achieved partial response or better (figure 1). The most common adverse events included thrombocytopenia, neutropenia, anemia, diarrhea, hypokalemia, fatigue and peripheral neuropathy. Adverse events of grade ≥3 included thrombocytopenia, neutropenia, anemia, elevated transaminase, diarrhea and pulmonary infection.
Conclusions: TQB3602 is generally well tolerated. The combination therapy has shown preliminary efficacy in RRMM, and further clinical trials are in progress. (NCT04275583)
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal