Abstract
Background Therapy-related leukemia is an increasingly recognized complication following improved treatment of and survival from various cancers. Therapy-related myeloid neoplasms are a well-established and understood entity and carry a poor prognosis. Therapy-related acute lymphoblastic leukemia (tALL), similarly, has been documented with incidence rates of 1-7%, but studies describing its features and response to treatment are limited. With the improved survival of patients with multiple myeloma (MM), the subsequent diagnosis of secondary malignancies is increasingly observed. Post MM tALL remains poorly described, however.
Methods We performed a retrospective review of adult patients diagnosed with tALL who were evaluated and treated at Princess Margaret Cancer Centre. We compared the presentation and clinical features of tALL post-MM to those of tALLs arising after other primary malignancies. We also explored prognostic variables including age, white blood cell (WBC) count, LDH, a prior diagnosis of MM, cytogenetics, type of induction chemotherapy, and allogeneic stem cell transplantation (alloSCT) as a time-dependent covariate.
Results The cohort included 65 patients from 1999 to 2022, of whom 18 (27.7%) had a previous diagnosis of MM. The cancer diagnosis prior to tALL was solid tumor, 34 patients (52.3%); hematologic, 30 patients (46.2%); and combined, 1 patient (1.5%). Multiple myeloma (18 patients, 27.7%) and breast cancer (13 patients, 20%) were the two most common prior malignancies. When compared to tALL following other cancers, patients with tALL after MM were older (64 years vs 53 years, p=0.009) and had distinct cytogenetic profiles (p=0.017). Median time to tALL diagnosis in the entire cohort was 5 years (0.0-26.0), and there was no difference between MM and other primary cancers. Time to diagnosis was numerically longer in tALL following other cancers versus MM, but this was not significant (Table 1). In multivariable analysis, age at ALL diagnosis (HR 1.04, 95% CI [1.01-1.07], p=0.025) influenced overall survival (OS) for all patients.
Focusing on the MM subgroup (n=18), all patients had B-ALL, and none had a BCR::ABL1 translocation or an MLL rearrangement. Front-line therapy for MM was cyclophosphamide, bortezomib, and dexamethasone (CYBORD) followed by autologous stem cell transplant (autoSCT) for all but one patient who received a tandem transplant. Six patients received prior radiation therapy for MM and all but one received lenalidomide as post autoSCT maintenance. The median interval between MM and tALL diagnoses was 4.83 years (3.1-10.4). Ten (55.6%) patients received pediatric-inspired induction chemotherapy (a modified Dana Farber Cancer Institute (DFCI) protocol). Three (16.7%) patients received upfront blinatumomab due to a previous diagnosis of severe neuropathy. Overall, thirteen (72.2%) patients achieved complete remission (CR) after induction chemotherapy, of which ten were residual disease (RD) negative. Three (16.7%) patients proceeded to alloSCT in first remission while one received alloSCT after salvage chemotherapy with blinatumomab. Seven (38.9%) patients have died (of which five died from sepsis).
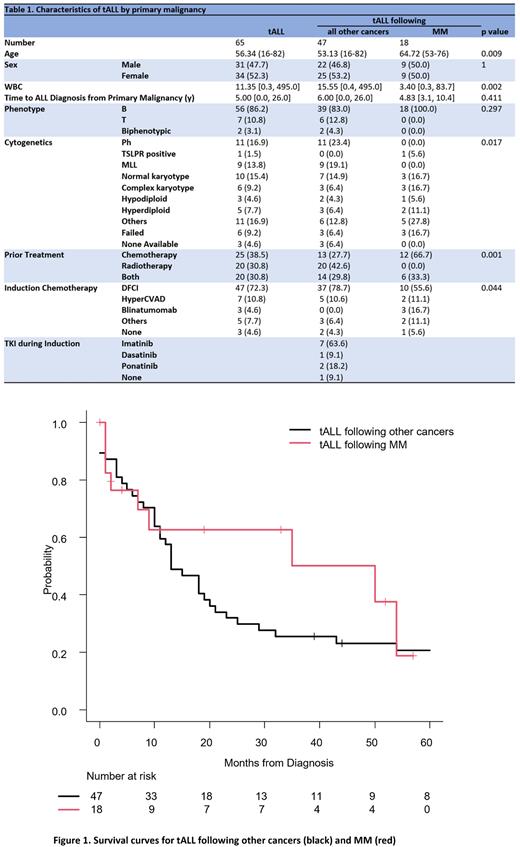
Median OS for tALL following all other cancers was 13 months overall (95% CI 10-20, p=0.332, and 25 months [95% CI 10-136, p=0.667] in the subset of those who received alloSCT. In contrast, the median OS for tALL following MM was 50 months overall (95% CI 2-NR), and 50 months (95% CI 7-NR) in those who received alloSCT (Figure 1). Cause of death was distinct between groups, with relapse of ALL more common in tALL following other cancers, and sepsis in tALL following MM (p=0.013). Relapse of prior MM was documented in four (22.2%) patients, and they all responded to salvage therapy.
Conclusion We reported a large cohort of patients with tALL, and the largest cohort of tALL following MM to our knowledge. The clinical features and genetic landscape in tALL following MM are distinct versus tALL arising after other cancers. Most patients achieved a complete remission and were RD negative following induction chemotherapy. The median OS of patients with post MM tALL is longer than that of tALL arising after other cancers. With the evolving changes in MM treatment patterns, further and continued evaluation of a larger cohort is recommended.
Disclosures
Gupta:Novartis: Consultancy, Honoraria; AbbVie: Consultancy, Other: Participation on a Data Safety or Advisory board; Pfizer: Consultancy, Other: Participation on a Data Safety or Advisory board; Roche: Other: Participation on a Data Safety or Advisory board; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Honoraria; BMS Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety or Advisory board; Sierra Oncology: Consultancy. Richard-Carpentier:AbbVie: Consultancy, Honoraria, Other: Advisory Board Participation; Taiho: Other: Advisory Board Participation; Astellas: Consultancy, Honoraria, Other: Advisory Board Participation; BMS: Other: Advisory Board Participation; Pfizer: Consultancy, Other: Advisory Board Participation. Schimmer:Novartis: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Otsuka Pharmaceuticals: Consultancy, Honoraria; UHN: Patents & Royalties: the use of DNT cells to treat AML; Medivir AB: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Takeda Pharmaceuticals: Consultancy, Honoraria, Research Funding. Schuh:Astellas: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; GlycoMimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Phebra: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Teva Pharmaceutical Industries: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees. Yee:Janssen: Research Funding; Forma Therapeutics: Research Funding; Geron: Research Funding; Astex: Research Funding; Abbvie: Honoraria; Takeda: Consultancy; TaiHo: Consultancy; Shattuck Labs: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Gilead: Research Funding; Karyopharm: Research Funding; Treadwell: Research Funding; Jazz: Consultancy, Research Funding; Bristol-Myers Squibb/Celgene: Consultancy; F. Hoffmann La Roche: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Astellas: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal