Abstract
Introduction: Patients (pts) with multiple myeloma (MM) frequently relapse requiring successive lines of therapy; those who experience early relapse (within 12 months [mo] of therapy initiation) have worse outcomes. The final progression-free survival (PFS) analysis of the Phase 3 IKEMA study (NCT03275285), performed 2 years after the prespecified interim analysis, confirmed that isatuximab (Isa) + carfilzomib (K) and dexamethasone (d) (Isa-Kd) significantly improved PFS compared with Kd in pts with relapsed MM (median PFS 35.7 [Isa-Kd] vs. 19.2 mo [Kd]; hazard ratio [HR] 0.58; 95.4% CI, 0.42-0.79), with a clinically meaningful increase in minimal residual disease negativity (MRD-) (33.5% vs. 15.4%) and complete response (CR) (44.1% vs. 28.5%) rates in the intent-to treat population, with a manageable safety profile. This subgroup analysis of IKEMA examined updated efficacy and safety of Isa-Kd vs. Kd in pts with MM who experienced early vs. late relapse.
Methods: Pts with 1-3 prior lines of therapy (LOT) were randomized 3:2 to receive Isa-Kd (n=179) or Kd (n=123). Treatment was given until progressive disease or unacceptable toxicity. These longer-term data at a median follow-up of 44 mo are based on a prespecified final PFS analysis of IKEMA; secondary endpoints included CR, MRD-, and safety. Early relapse pts included those who relapsed <12 mo from initiation of the most recent LOT for pts with ≥2 prior LOT, <18 mo for pts with 1 prior LOT, and <12 mo from autologous stem-cell transplantation (ASCT). Late relapse was defined as pts who relapsed ≥12 mo from initiation of the most recent LOT for those with ≥2 prior LOT and ≥18 mo for pts with 1 prior LOT.
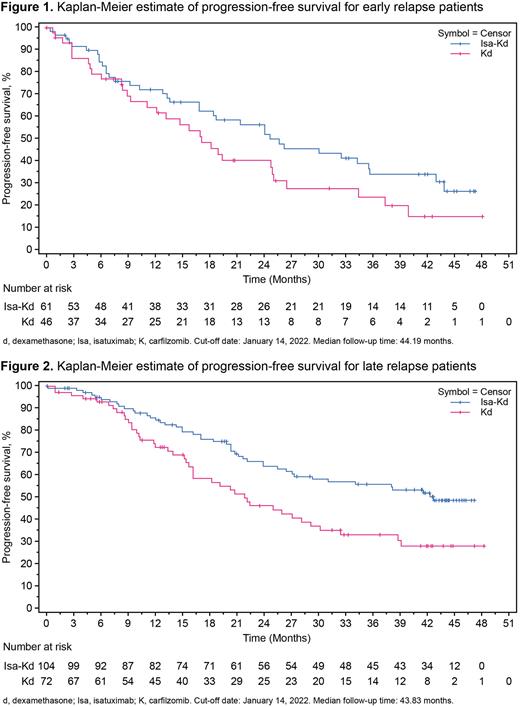
Results: There were 107 early relapse pts (61/179 [34.1%] Isa-Kd, 46/123 [37.4%] Kd) and 176 late relapse pts (104 [58.1%] Isa-Kd, 72 [58.5%] Kd. Among early relapse pts, the Isa-Kd arm had a higher proportion of pts who had renal impairment (31.0% vs. 15.4%) and lower incidence of pts with ISS Stage I (31.1% vs. 54.3%) compared with the Kd arm. The median number of prior LOT was 2 for both treatment arms in early relapse pts and in the late relapse Kd arm; late relapse Isa-Kd arm had a median of 1 prior LOT (55.8% had 1 prior LOT with Isa-Kd vs. 48.6% pts with Kd). In late relapse, more pts had the chromosomal abnormality 1q21+ with Isa-Kd (44.2%) vs. Kd (33.3%). At data cut-off, the median PFS was longer for pts treated with Isa-Kd vs. Kd in both early relapse (24.7 vs. 17.2 mo; HR 0.662 [95.4% CI, 0.404-1.087]) and late relapse (42.7 vs. 21.9 mo; HR, 0.542 [95.4% CI, 0.353-0.833]) pts (Figures 1 and 2). The overall response rates were 82.0% vs. 82.6% in early relapse pts, and 90.4% vs. 86.1% in late relapse pts with Isa-Kd vs. Kd, respectively. More pts achieved very good partial response or better (early relapse: 67.2% vs. 52.2%; late relapse: 76.0% vs. 58.3%), MRD- (early relapse: 24.6% vs. 15.2%; late relapse: 37.5% vs. 16.7%), and MRD- CR rates (early relapse: 18.0% vs. 10.9%; late relapse: 30.8% vs. 13.9%) with Isa-Kd vs. Kd, respectively. Grade ≥3 and serious treatment-emergent adverse events (TEAEs) were similar in both treatment arms in early relapse pts, but were higher in the Isa-Kd arm in late relapse pts; however, rates of TEAEs leading to definitive discontinuation or death were similar in both treatment arms across both early and late relapse pts. All-grade TEAEs reported more frequently with Isa-Kd (≥10% difference vs. Kd) included infusion reactions in early relapse (41.0% vs. 6.5%) and late relapse pts (50.0% vs. 1.4%), upper respiratory tract infection (38.2% vs. 26.8%), fatigue (32.4% vs.19.7%), dyspnea (36.3% vs. 22.5%), bronchitis (30.4% vs. 12.7%), cough (23.5% vs. 11.3%), and gastroenteritis (14.7% vs. 4.2%) in late relapse pts. Hematologic laboratory abnormalities reported more frequently (≥5%) in the Isa-Kd arm included Grade 3 anemia (42.6% vs. 30.4%), Grade 3 neutropenia (18.0% vs. 4.3% ), and Grade 3 and 4 thrombocytopenia (21.3% vs.15.2% and 18.0% vs 13.0%) in early relapse pts and Grade 3 neutropenia (13.7% vs. 8.5%) in late relapse pts with Isa-Kd vs. Kd, respectively.
Conclusions: The addition of Isa to Kd improved PFS and depth of response, with a manageable safety profile in both early and late relapse pts, consistent with the benefit observed in the overall IKEMA study population. These results support Isa-Kd as a standard of care in pts with relapsed and/or refractory MM regardless of early or late relapse.
Funding: Sanofi
Disclosures
Moreau:AbbVie, Janssen, Celgene, Amgen, and Sanofi: Honoraria. Baker:AbbVie: Research Funding; Acerta Pharma: Research Funding; Alexion: Research Funding; Amgen: Research Funding; Bayer: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Boehringer Ingelheim: Research Funding; Celgene: Research Funding; CSL Behring: Research Funding; Daiichi Sankyo: Research Funding; Jansen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MorphoSys: Research Funding; Pfizer: Research Funding; Portola: Research Funding; Rigel Pharmaceuticals: Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Takeda: Research Funding; Pharmaxis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biegene: Research Funding; Technoclone: Research Funding; Cardinal Health: Honoraria. Leleu:Janssen: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; BMS: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria. Mohty:Celgene: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Takeda: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Novartis: Honoraria; GSK: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria. Karlin:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Rawlings:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Tekle:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Risse:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Martin:Amgen, Johnson & Johnson / Janssen, Sanofi, and Seattle Genetics: Research Funding; GlaxoSmithKline and Legend Biotech: Consultancy; Legend Biotech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal