Abstract
Background:
High-dose methotrexate (HD-MTX) is considered to be beneficial for newly diagnosed PCNSL; however, the standard of care in the front line has not been determined yet (Yuan et al., Biomark Res 2021). Bruton's tyrosine kinase (BTK) inhibitors have shown encouraging results in both recurrent/refractory (R/R) and newly diagnosed PCNSL (Grommes et al, Blood 2019; Guo et al, ASH 2021). Orelabrutinib is a potent, irreversible, high selectivity BTK inhibitor with fewer off-target effects (Dhillon, Drugs 2021) and a high concentration in the cerebrospinal fluid (Zhang et al, EHA 2022). Recently, it's reported that orelabrutinib showed encouraging efficacy and safety profiles in PCNSL (Wu et al., Invest New Drugs 2022). In addition, both anti-programmed cell death protein-1 (PD-1) antibody and fotemustine-based therapy had preliminary efficacy in PCNSL (Nayak et al., Blood 2017; Wu et al., J Neurooncol 2018). Thus, a phase I/II study (NCT04831658) was designed to evaluate the safety and efficacy of the combination of orelabrutinib, anti-PD-1 antibody, and fotemustine in patients (pts) with newly diagnosed PCNSL. Herein, we reported the results from the phase I study (dose-escalation stage), of which the primary objective was to determine the recommended phase II dose (RP2D) of orelabrutinib when combined with anti-PD-1 antibody and fotemustine.
Methods:
In this ongoing, prospective, phase I/II study, pts aged ≥18 years with newly diagnosed PCNSL were eligible. In phase I, pts received escalating doses (100 mg, 150 mg, and 200 mg) of daily oral orelabrutinib in a 3+3 design, with a fixed dose of 200 mg anti-PD-1 antibody on day 1 and 100 mg/m2 fotemustine on day 2 of a 21-day cycle. The primary endpoint was dose-limiting toxicity (DLT). In phase II, pts received the combination at the RP2D for 6 cycles, followed by the maintenance therapy. The maintenance therapy was administrated according to the tumor response of the induction therapy, including the orelabrutinib (3 months) plus anti-PD-1 antibody (1 year) for the complete responders, the orelabrutinib (3 months), anti-PD-1 antibody (1 year) plus whole-brain radiotherapy (WBRT) for the partial responders. Pts with stable or progressive disease who required withdrawal from the study received salvage therapy. In phase II, the primary endpoint was objective response rate (ORR), and the secondary endpoints included disease control rate (DCR), progression-free survival (PFS), overall survival (OS), time to progression, median survival time, safety, and quality of life. AEs were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Quality of life was assessed using EORTC-QLQ-C30.
Results:
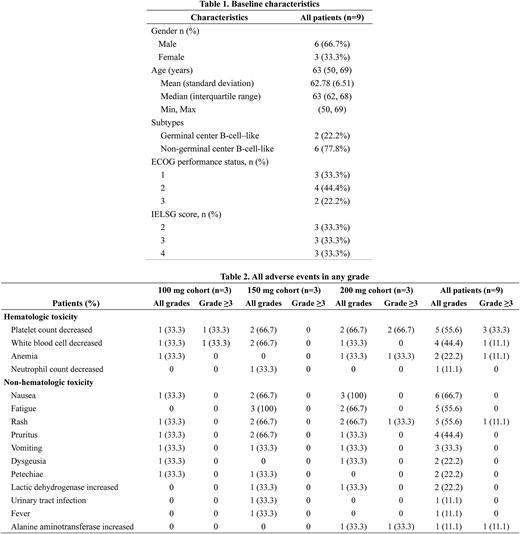
As of June 9, 2022, the dose-escalation phase was completed and enrolled 9 pts with a median age of 63 years (range: 50-69), median ECOG performance score of 2 (range: 1-2), and median IELSG score of 3 (range: 2-4, Table 1). Seven (77.8%) pts had the non-germinal center B-cell-like (non-GCB) subtype by Hans criteria. In the dose-escalation cohorts (n=9), no DLTs were observed up to the maximum proposed dose of 200 mg daily. The RP2D of orelabrutinib was determined to be 150 mg based on tolerability and preliminary efficacy. The median duration of treatment was 157 days (IQR, 139-425). Adverse events (AEs) of any grade occurred in 8 (88.9%) of 9 pts (Table 2). The common AEs irrespective of causality were nausea (66.7%), platelet count decreased (55.6%), fatigue (55.6%), and rash (55.6%). The most common grade 3/4 AE was platelet count decreased (33.3%). Two (22.2%) pts in the 200 mg cohort experienced orelabrutinib dose reductions due to platelet count decreased. No treatment-related death was reported. As assessed by investigators, the ORR was 88.9% (8/9) including 2 (22.2%) CR, 1 (11.1%) CR unconfirmed (CRu), and 5 (55.6%) partial response while 1 (11.1%) pt had progressive disease. One pt (150 mg cohort) achieved PR after 2 cycles of treatment and then further achieved CR from CRu at the end of the sixth cycle. As of the cut-off date, PFS and OS were immature.
Conclusion:
Orelabrutinib was well-tolerated when combined with anti-PD-1 antibody and fotemustine in the current study. The encouraging results of this combination were observed in the pts with newly diagnosed PCNSL. Phase 2 is actively recruiting pts; more response and toxicity data will be presented.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal