Abstract

Introduction BCNU containing BEAM (BCNU, etoposide, cytarabine, melphalan) is one of the most commonly used conditioning regimens in lymphoma patients before autologous stem cell transplantation (ASCT). A frequently observed non-hematological complication of high-dose BCNU containing regimens is pulmonary toxicity that might lead to permanent damage with a reported incidence varying from 2% to 64%. Replacing BCNU with bendamustine in the BeEAM regimen has shown to be effective and feasible in phase II trials. So far, no randomized clinical trial has compared the established BEAM protocol with the BeEAM regimen. Here we present the results of a randomized phase II clinical trial (EudraCT2014-003629-16).
Methods In this multicentre Austrian Swiss trial, 108 patients were enrolled from January 2015 to December 2018 and randomly assigned to the BeEAM (N=53) or BEAM (N=55) arm as a conditioning regimen. Patients enrolled had either mantle cell lymphoma (MCL) (N=39), diffuse large B-cell lymphoma (DLBCL) (N=57) or follicular lymphoma (FL) (N=12). The conditioning regimen was administered according to the standard BEAM protocol (BCNU: 300mg/m2) and the BeEAM regimen was given according the regimen first published by Giuseppe Visani et al. 2011 (bendamustine: 2x200mg/m2 on consecutive days). Hematological engraftment after ASCT was defined as the first day of neutrophil counts above 0.5 x 109/l, and of platelet counts above 20 x 109/l in the absence of platelet transfusion in the previous 3 days. The primary endpoint of the study was to evaluate whether replacement of BCNU by bendamustine could reduce lung toxicity defined, as a decrease of the diffusion capacity of the lung for carbon monoxide (DLCO) by at least 20%, at 3 months after ASCT.
Secondary endpoints included overall survival (OS) and progression-free survival (PFS), acute toxicity, hematological recovery and engraftment, pulmonary function parameters as assessed by spiro- and ergometry from diagnosis until 1-year after ASCT and quality of life according to the EORTC-Q30 questionnaire.
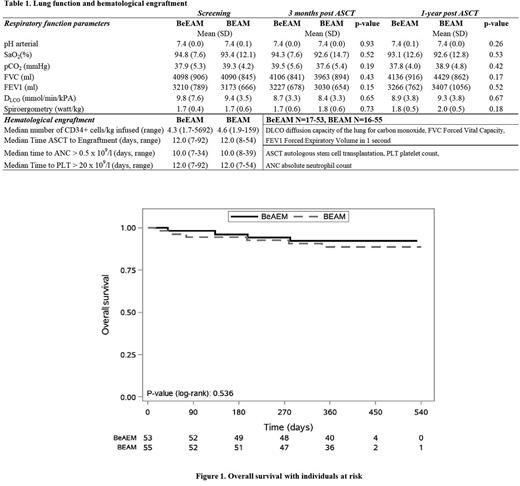
ResultsAge, disease status and remission status were well balanced and did not differ between the two study groups. Engraftment: Median number of infused CD34+ cells x 106/kg was 4.3 in the BeEAM vs. 4.6 in the BEAM arm. All patients engrafted and there was no difference in time to engraftment between the BeEAM and BEAM group.
Respiratory function: The primary endpoint was achieved in 8/41(19.5%) in the BeEAM and in 9/43 (20.9%) in the BEAM group. There was no significant difference in DLCO and spiro- and ergometry in the BeEAM and BEAM group during the whole study period (Table 1). Although 2 patients died because of treatment related lung toxicity in the BEAM arm, we did not observe any functional detoriation in the patients who received the BCNU-containing BEAM regimen. Additionally, blood gas analysis revealed similar results in both study arms at screening, 3 months and 1-year after ASCT.
Toxicity: Acute toxicity grade 3-5 according to CTCAE v4.0 was observed in 43.4% of the patients in the BeEAM arm and in 34.5% of the patients in the BEAM arm in the category any adverse event (p =0.43). Infections and infestations were observed in 9.4% of patients in the BeEAM arm and in 14.5% in the BEAM arm (p=0.56). Respiratory, thoracic and mediastinal disorders were only detected in the BEAM arm (5.5%) (p=0.24). In contrast to previous trials, grade 3-5 renal toxicity was low, both in the BeEAM and BEAM arm, respectively (1.9% vs. 1.8%) (p=1.00). Gastrointestinal toxicity occurred more frequently in the BeEAM arm (13.2%) than in the BEAM arm (5.5%) (p=0.20). Median follow-up was 369 days (15-540 days). In the BeEAM arm 4 out of 53 patients died during the observational period, 6 out of 55 in the BEAM arm (log-rank test p=0.54, Figure 1). Ten patients in each group had a progression or died during the observational period (log-rank test p=0.89).
Quality of life: There was no statistical difference in the assessment of quality of life in the two study arms.
Conclusion BeEAM is a feasible, well tolerated regimen resulting in similar clinical results as observed with the BEAM regimen. Interestingly we did not see any difference in impairment of pulmonary function, however 2 patients died in the BEAM arm of lung toxicity and no lung related deaths were observed in the BeEAM group. In our conclusion, BeEAM is a reasonable alternative to the BEAM regimen.
Disclosures
Keil:Novartis: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Astra Zeneca: Honoraria; Abbvie: Honoraria, Research Funding; Roche: Honoraria; Takeda: Honoraria, Research Funding; BMS: Honoraria; Incyte: Honoraria. Müller:Novartis: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Buxhofer-Ausch:Novartis: Consultancy, Honoraria; Kite-Gilead: Consultancy, Honoraria; Celgene-BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Noesslinger:Janssen: Honoraria; BeiGene: Honoraria; Roche: Honoraria; Abbvie: Honoraria; Astra Zeneca: Honoraria; Gilead: Honoraria; Celgene: Honoraria. Greil:Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; MSD Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; BMS-Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Hoffmann - La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal