Abstract
Introduction: Pediatric acute myeloid leukemia (AML) is characterized by significant cytogenetic and molecular heterogeneity. AML with CBFA2T3::GLIS2 fusion (CBF::GLIS) involving infants and very young children is a rare but uniformly refractory disease with high mortality. Laboratory data demonstrates that this fusion is sufficient for leukemic transformation without recurrent secondary variants. These studies have also linked CBF::GLIS fusion to induction of folate receptor alpha (FOLR1) expression in transformed cells (Meshinchi et al, 2020). Incidence of Ph+ de novo (BCR::ABL minor breakpoint, p190 hybrid) disease ranges from 0.5-3% of all AML cases across the age spectrum (Soupir et al, 2017). We, for the first-time, report dual existence of both fusions in a child with AML and discuss novel interventions including combination of a BCR::ABL tyrosine kinase inhibitor (TKI) and antifolate therapy consisting of methotrexate (MTX) and FLOR1-specific antibody-drug conjugate (ADC) STRO-002 (Sutro Biopharma) based on available data regarding its in-vitro preclinical activity in CBF::GLIS disease (Meshinchi et al, ASH abstract, 2021).
Case: A 2-year-old girl of Vietnamese descent received a bone marrow examination for bicytopenia and macrocytosis. Flow cytometry confirmed the poor prognostic RAM immunophenotype AML (CD45 dim, HLA-DR negative, CD38 dim, CD56 bright) and FOLR1 expression. Marrow aspirate smears showed numerous hypercellular spicules and blasts with scant cytoplasm and some blebbing but no granules or Auer rods. Marrow exhibited 100% cellularity. CNS was not involved. Review of diagnostic genomic profile mid induction showed a BCR::ABL minor breakpoint fusion as well as CBF::GLIS fusion.
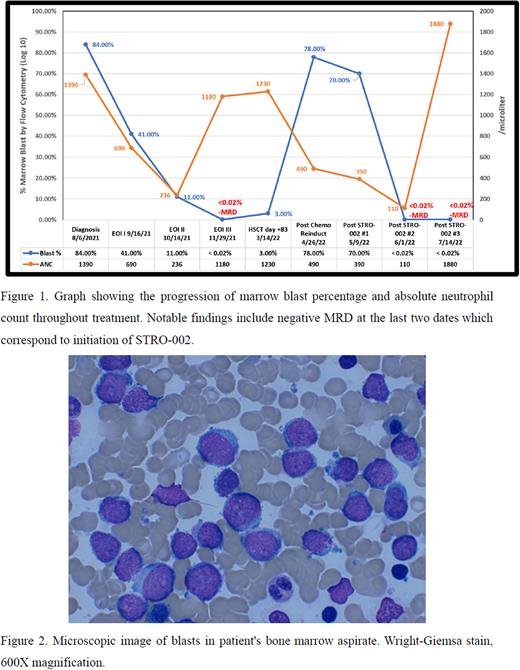
The child began AML induction on COG AAML1831 trial randomized to the experimental arm with CPX-351 and gemtuzumab ozogamicin (GO). Due to lack of response to AML therapy at end of Induction (EOI) I, she was taken off protocol and started on modified ALL regimen for Induction II consisting of cytarabine, low dose weekly methotrexate and bimonthly peg-asparaginase with addition of an oral TKI, dasatinib. EOI II marrow examination showed improvement with reduction in blasts (11%) and detectable BCR::ABL but undetectable CBF::GLIS by Q-RT-PCR. Induction III included low dose weekly methotrexate, cytarabine, GO, and dasatinib. EOI III marrow examination showed complete morphologic remission (CR) with no evidence of minimal residual disease (MRD) or fusion transcripts of either clone.
Due to availability of a matched sibling donor, the child underwent hematopoietic stem cell transplantation (HSCT); however, was noted to have 3% blasts on day +83 marrow examination despite previously commencing dasatinib post-transplant prophylaxis. BCR::ABL and CBF::GLIS transcripts were again detectable at low levels. Dasatinib dosage was maximized and weekly MTX and bimonthly peg-asparaginase were reintroduced, but the marrow disease burden increased significantly to 78% blasts positive only for CBF::GLIS fusion. Given availability of FOLR1 directed ADC on single-patient compassionate use basis, patient received single agent STRO-001 on a bi-monthly basis. With rising ANC, evaluation of marrow after 2nd STRO-002 dose remarkably showed CR with MRD negative remission by flow and molecular studies. This time remission was consolidated with monthly STRO-002 and donor lymphocyte infusions (DLI). Currently, patient remains in MRD-negative remission after the 3 of 4 planned STRO-002/DLI maintenance. Patient has maintained a 100% Lansky play score and tolerated the regimen with only prolonged initial cytopenia requiring supportive care as a toxicity. Plan is to continue TKI and monthly STRO-002 as maintenance therapy.
Conclusion: Complete genomic sequencing has revolutionized pediatric AML categorization. It is quite conceivable that (BCR::ABL, CBF::GLIS) dual fusion disease will become a more common finding in future. Although CBF::GLIS AML is uniformly refractory to conventional therapy, targeted therapy with FOLR1-directed STRO-002 was highly effective in eliminating CBF::GLIS clone in this patient, and combination with DLI appears to be feasible. Long-term follow-up is required to assess durability of remission. Additional testing of this approach in a larger patient population is needed to determine the role of STRO-002 in this high-risk pediatric AML population.
Disclosures
Molina:Sutro Biopharma: Current Employment, Current equity holder in publicly-traded company. Eidenschink Brodersen:Hematologics Inc.: Current Employment, Current equity holder in private company. Hudson:Hematologics, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal