In this issue of Blood, Keyvani Chahi et al1 have described the transcription factor PLAG1 as a positive regulator of human cord blood (CB) hematopoietic stem cell (HSC) dormancy and self-renewal by repressing the expression of translational machinery that is customarily activated in response to ex vivo cultivation stress.
HSCs are capable of generating all blood cell lineages throughout a person’s lifetime.2 To preserve life-long stem cell functionality, HSCs primarily reside in a state of dormancy and quiescence characterized by low levels of protein synthesis.3,4 Under stress conditions, such as ex vivo cultivation or transplantation, dormant HSCs are forced into a state of activation.5 However, little is known about the preventative machinery that suppresses activation and promotes quiescence in human HSCs.
Previously, PLAG1 was shown to bind to the promoter of MSI2 to enhance in vitro expansion of hematopoietic stem and progenitor cells (HSPCs).6 In their study, Keyvani Chahi et al showed that PLAG1 expression is restricted to non-cycling HSCs, which suggests a role for PLAG1 in modulating human HSPC function that is independent of the PLAG1/USF2-MSI2 regulatory axis. There are 3 human PLAG1 transcript variants encoding 2 protein isoforms. The shorter isoforms of PLAG1 (B and S) are enriched in CB HSCs, while the longer isoform (A) is expressed at much lower levels.6 Knockdown of PLAG1 reduced the output of human HSPCs and was accompanied by impaired long-term bone marrow reconstitution, whereas the ectopic overexpression (OE) of the PLAG1-S isoform enhanced in vivo self-renewal of human HSCs.
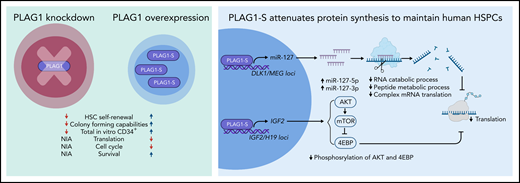
From genomic binding and transcriptomic profiling of PLAG1-SOE HSPCs, the authors found that PLAG1-S can act as both a positive and a negative regulator of gene expression. For instance, ribosomal protein expression was downregulated in PLAG1-SOE, which pointed toward the ability of PLAG1-S to attenuate protein synthesis machinery and thus regulate stem cell fate decisions. In agreement with this finding, the authors showed that PLAG1-SOE maintained low global translation rates in ex vivo cultivated HSPCs (see figure).
Loss and gain of PLAG1 function in human CB CD34+ HSPCs (left). Mechanisms by which PLAG1-S represses protein synthesis to maintain human CB HSC dormancy and self-renewal (right). mRNA, messenger RNA; NIA, no information available. Figure created by BioRender.com.
Loss and gain of PLAG1 function in human CB CD34+ HSPCs (left). Mechanisms by which PLAG1-S represses protein synthesis to maintain human CB HSC dormancy and self-renewal (right). mRNA, messenger RNA; NIA, no information available. Figure created by BioRender.com.
Mechanistically, PLAG1-S directly binds and activates the imprinted loci IGF2/H19 and DLK1/MEG3. IGF2 stimulates the PI3K-AKT-mTOR signaling pathway, whose activation in turn facilitates protein synthesis. Here, phosphorylated AKT activates mTOR to phosphorylate 4EBP. In the phosphorylated state, 4EBP cannot inhibit protein synthesis. However, intracellular flow cytometry revealed reduced phosphorylation of AKT and 4EBP, which suggests that PLAG1-S represses phosphorylation of 4EBP and thus contributes to reduced protein synthesis. Indeed, the pharmacological inhibition of AKT and mTOR decreased total cell count but increased the proportion of HSPCs. However, only mTOR inhibition, but not AKT inhibition, attenuated protein synthesis. The DLK1/MEG3 locus encodes four microRNAs (eg, miR-127)7 that were upregulated in PLAG1-SOE. The targets of these miRNAs included genes involved in complex cap-dependent translation and RNA and peptide metabolic processing, which were downregulated in PLAG1-SOE. In the presence of an inhibitory miR-127-5p sponge, PLAG1-SOE reduced HSPC output and increased protein synthesis, whereas overexpression of miR-127 enhanced HSPC output and reduced protein synthesis (see figure).
Intriguingly, MYC expression, a major transcriptional activator of cytoplasmic translation and ribosome biogenesis,8 was not reduced in PLAG1-SOE. PLAG1-SOE instead upregulates MYC-regulated nuclear ribosome assembly targets, suggesting that PLAG1-S acts independently of MYC repression. PLAG1-SOE in the presence of c-MYCOE was able to reduce protein synthesis and increase HSPC output, which was previously negatively impacted by c-MYCOE.
Keyvani Chahi et al identified PLAG1-S as a novel enforcer of human CB HSC dormancy and self-renewal via its dampening of protein synthesis upon ex vivo culture-induced stimulation. These results further highlight the physiological importance of low translation rates and suggest targeting proteostasis to manipulate ex vivo human CB HSCs for therapeutic benefits (eg, gene therapy). Furthermore, it has been demonstrated that translational inhibitors can eliminate primitive leukemic cells while sparing healthy HSCs.9 Targeting components of the PLAG1-S signaling pathway could provide novel therapies for patients with leukemia.
It is worth highlighting the great effort the scientific community is making to understand how human stem cells are regulated, which will pave the way for the discovery of new ways to maintain and genetically modify these rare and precious blood cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal