In this issue of Blood, Cuvelier et al1 show that outcomes for hematopoietic stem cell transplantation (HSCT) have improved and compete favorably with ex vivo gene therapy (GT) for patients with adenosine deaminase-deficient severe combined immunodeficiency (ADA-SCID).
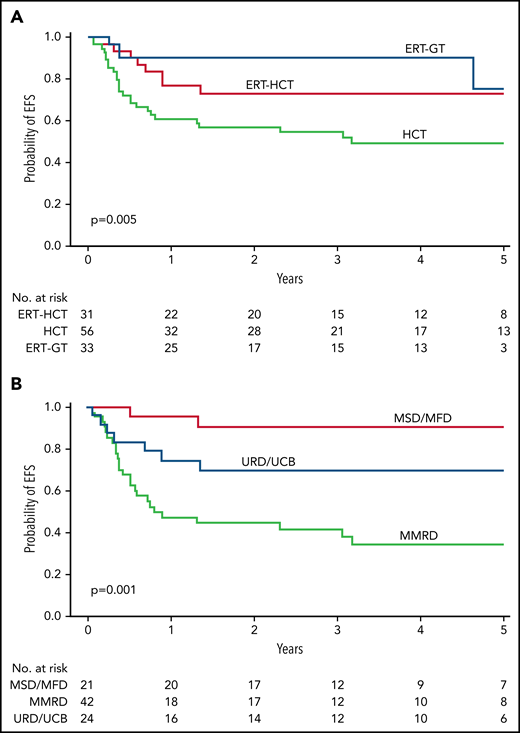
Cuvelier and colleagues comprehensively report treatment outcomes for a cohort of 131 children with ADA-SCID. The analysis from the 26 Primary Immunodeficiency Treatment Consortium member centers in the United States and Canada covers an impressive 35-year time period. Cuvelier and colleagues found that HSCT with a matched sibling donor (MSD), a matched unrelated donor (MUD), or cord blood (CB) gave very good results. HSCT with an MSD resulted in essentially the same results as GT, although the direct comparison is really not valid, as discussed below.
ADA-SCID is a multisystem disease caused by a defect in purine metabolism. The patient experiences not only early symptoms because of the profound impairments in the adaptive and innate immune systems but also a wide range of nonimmune complications in the pulmonary, hematological, gastrointestinal, neurological, and skeletal organ systems.2 This notably includes damage to the pulmonary and gastrointestinal epithelia through the accumulation of toxic metabolites; this damage explains why it is so difficult to develop an acceptable conditioning regimen in this form of SCID.
The case series by Cuvelier and colleagues is the largest reported to date. It included 4 different treatment groups: enzyme replacement (ERT) alone (n = 9), HSCT preceded by ERT (n = 31), HSCT not preceded by ERT (n = 56), and ex vivo GT with a gammaretroviral or lentiviral vector (n = 33, after ERT in all cases). For patients having an HSCT from an MSD after the year 2000, the 5-year overall survival (OS) rate was 100%, and the event-free survival (EFS) rate was 95%; the equivalent rates for lentivirus-mediated GT were 100% and 75%, respectively. However, this attempt to compare outcomes for HSCT vs GT has a major issue: overall, the patients in the GT group were younger than those in the HSCT group and did not have ongoing infections at the time of conditioning. It is well known that OS and EFS rates are lower when the patient with SCID has an active infection at the time of HSCT.3 Despite this limitation, GT with a lentiviral vector gave excellent results and had some important advantages over GT with first-generation gammaretroviral vectors. In patients treated with a lentiviral vector, the engraftment rate was significantly higher, and the immune reconstitution was significantly more robust (probably because of the vector's greater ability to correct HSCs). Despite the small number (n = 12) of patients in the retroviral vector group, which precludes a robust comparison, the excellent results associated with lentiviral GT should now prompt us to find a way of making this approach affordable and sustainable for all patients who lack an MSD.
The use of ERT before the infusion of gene-modified autologous cells did not appear to blunt the putative selective advantage of ADA-corrected cells and was not associated with a significantly higher incidence of autoimmune adverse events. Likewise, the use of ERT before HSCT did not increase graft failure due to resistance. There are ≥3 possible mechanistic explanations for the improved outcomes of definitive stem cell therapy after ERT: (i) partial correction of hypocellularity within the bone marrow (which results in the more effective mobilization of HSCs for use in GT), (ii) a shorter period of lymphopenia (ie, before the production of new lymphocytes by the gene-corrected graft), and (iii) protection against systemic toxicity (particularly damage to the thymic and pulmonary epithelia).4 Nevertheless, Cuvelier and colleagues did not find a significant, positive impact of prior ERT on the outcome of HSCT. Hence, further prospective studies are required before we can draw any definitive conclusions about the optimal timing and duration of ERT before curative cell therapy.
The OS rate after HSCT was lower with a MUD/CB (78.5%) than with an MSD, except, of course, for patients without an active infection. Moreover, Cuvelier and colleagues found that HSCT with a mismatched related donor (MMRD) gave significantly worse results, thus further confirming the findings of the European registry study (see figure).3,5 However, the data from Cuvelier and colleagues are difficult to interpret because various conditioning regimens and T-cell depletion methods were used over a very long analysis period (covering more than 4 decades); hence, the value and positioning of this donor source cannot be determined. We shall have to wait and see whether the introduction of new cell therapy approaches and less toxic conditioning regimens will significantly change the prognosis for HSCT with an MMRD.6,7
EFS and OS for ADA-SCID. (A) Five-year EFS by FDCT. (B) Five-year EFS for all transplant patients (including those with and without a preceding period of ERT) by donor type. HCT, Hematopoietic cell transplantation; MFD, Matched familial donor; URD/UCB, unit cord blood. See Figure 2A,C in the article by Cuvelier et al that begins on page 685.
EFS and OS for ADA-SCID. (A) Five-year EFS by FDCT. (B) Five-year EFS for all transplant patients (including those with and without a preceding period of ERT) by donor type. HCT, Hematopoietic cell transplantation; MFD, Matched familial donor; URD/UCB, unit cord blood. See Figure 2A,C in the article by Cuvelier et al that begins on page 685.
There is an urgent need for novel immunotherapies that accelerate and augment immune reconstitution in ADA-SCID because infections are still the main cause of transplant-related morbidity and mortality (except in the more highly selected GT group). Moreover, the T-cell production reported by Cuvelier and colleagues was suboptimal and varied greatly from one patient to another. An ongoing European trial in SCID patients transplanted with an MMRD (the setting with the lowest clinical improvements) has been designed to determine whether add-on injections of T-cell precursors can significantly improve T-cell reconstitution.8,9 In the setting of ADA-SCID (where toxic damage affects the thymic epithelial cells), the use of growth factors to rejuvenate the epithelium might also be a valuable strategy.10 Lastly, there is a huge clinical benefit provided by newborn screening programs for SCID; the diagnosis of these immunodeficiencies soon after birth (ie, before the onset of infections) might enable successful HSCT even in patients lacking an MSD or when GT is not available.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal