TO THE EDITOR:
Despite several advances in strategies to control the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including rapid vaccine development and deployment, as well as antiviral therapeutics, the emergence of new variants continue to lead to significant surges of disease globally.1 Experts predict the virus will likely remain endemic in the future.2 In December 2021, the antiviral Paxlovid (nirmatrelvir-ritonavir [NMV/r]) was granted authorization for the treatment of patients with mild to moderate coronavirus disease 2019 (COVID-19) in the ambulatory setting. Among high-income countries, ∼1% to 2% of the population is prescribed long-term anticoagulants,3 with a large number of individuals receiving ongoing warfarin therapy.4,5 A common challenge with using warfarin is a narrow therapeutic window that requires close international normalized ratio (INR) monitoring. Because of the metabolism of warfarin, drug-drug interactions can cause considerable INR variability.6,7 Ritonavir is a potent inhibitor and inducer of key drug-metabolizing enzymes, and thus, it has the potential to interact with other drugs that are metabolized through this key pathway, including warfarin.8
Ritonavir, which is a strong cytochrome P450 3A4 (CYP3A4) inhibitor and a P-glycoprotein inhibitor, is coadministered with nirmatrelvir to boost the blood concentration of nirmatrelvir, thereby making it effective against SARS-CoV-2.9 The half-life of ritonavir is 6.15 hours and that of nirmatrelvir when coadministered with ritonavir is 6.05 hours. Vitamin K antagonists often consist of different active enantiomers that vary in their activity and pharmacokinetic profiles. The S-enantiomer of warfarin is more potent and predominantly metabolized by CYP2C9, whereas the less active R-enantiomer is metabolized by CYP3A4 and CYP1A2.10 Ritonavir is an inhibitor of CYP3A4 but can induce CYP2C9 and CYP1A2.11 Thus, coadministration of ritonavir with warfarin can have variable effects on the efficacy of warfarin. The onset of CYP inhibition occurred more rapidly than that of CYP induction. Thus, although long-term use of ritonavir (as seen in antiretroviral therapy) has been reported to reduce the effect of warfarin,12,13 it is possible that a shorter 5-day exposure to NMV/r could lead to the opposite effect.14 Guidance documents recommend caution and close monitoring owing to potential interactions based on preclinical data that warfarin is metabolized by several CYPs, which are inhibited or induced by the antiviral. Owing to the widespread use of NMV/r for the management of COVID-19 and the fact that its impact on INR levels remains unknown, we evaluated the INR levels in a series of patients treated with NMV/r and warfarin.
We identified patients currently registered at the Beth Israel Deaconess Medical Center (Boston, MA) and Lahey Medical Center (Burlington, MA) ambulatory warfarin clinics who were diagnosed with COVID-19 after December 2021 and who received NMV/r for antiviral therapy. The study was exempted from review by the institutional review boards of both the institutions. Relevant patient information, including the duration of warfarin use, indication for anticoagulation, target INR goal, date of COVID-19 diagnosis, and timing of NMV/r initiation was also collected. INR values from up to 3 months before data collection (1 June 2022) were also collected. Details of any bleeding or thrombotic events (if any) after the diagnosis of COVID-19 were manually extracted from the electronic medical record.
A total of 29 patients were identified during the study period (Table 1). The median patient age was 70 years (IQR, 51-90 years), and 12 patients (41%) were female. The most common indication for warfarin therapy was atrial fibrillation (45%), followed by mechanical heart valves (17%) and venous thromboembolism (17%). Almost all patients were treated with a target INR of 2 to 3 (90%). The median time in the therapeutic range was 76% (IQR, 36%-100%). The median INR before administration of NMV/r (range, 1-33 days) was 2.4 (IQR, 1.1-3.7).
Clinical characteristics of patients receiving MNV/r while on chronic warfarin therapy
| . | N = 29 . |
|---|---|
| Age, median (IQR), y | 70 (51-90) |
| Sex, n (%) | |
| Female | 12 (41.4) |
| Male | 17 (58.6) |
| Indication for warfarin, n (%) | |
| Atrial fibrillation | 14 (48.3) |
| Venous thromboembolism | 7 (24.1) |
| Mechanical heart valve | 6 (20.6) |
| Other∗ | 2 (6.9) |
| Target INR, n (%) | |
| 1.5-2.5 | 1 (3.4) |
| 2.0-3.0 | 26 (89.6) |
| 2.5-3.5 | 2 (6.9) |
| Time in therapeutic range for prior 12 months, median (IQR) | 74 (36-100) |
| INR before start of NMV/r, median (IQR) | 2.4 (1.1-3.7) |
| INR after start of NMV/r, median (IQR) | 1.95 (1.5-2.3) |
| . | N = 29 . |
|---|---|
| Age, median (IQR), y | 70 (51-90) |
| Sex, n (%) | |
| Female | 12 (41.4) |
| Male | 17 (58.6) |
| Indication for warfarin, n (%) | |
| Atrial fibrillation | 14 (48.3) |
| Venous thromboembolism | 7 (24.1) |
| Mechanical heart valve | 6 (20.6) |
| Other∗ | 2 (6.9) |
| Target INR, n (%) | |
| 1.5-2.5 | 1 (3.4) |
| 2.0-3.0 | 26 (89.6) |
| 2.5-3.5 | 2 (6.9) |
| Time in therapeutic range for prior 12 months, median (IQR) | 74 (36-100) |
| INR before start of NMV/r, median (IQR) | 2.4 (1.1-3.7) |
| INR after start of NMV/r, median (IQR) | 1.95 (1.5-2.3) |
Includes a patient each with antiphospholipid antibody syndrome and intracardiac thrombosis.
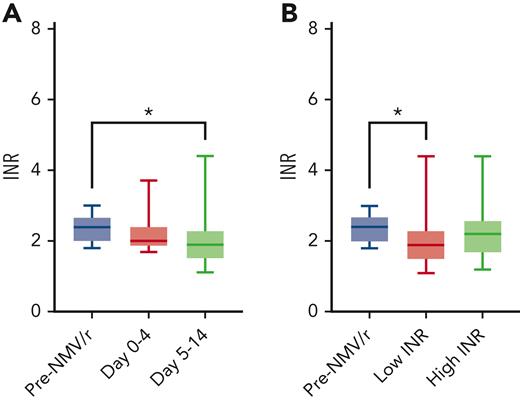
None of the included patients had any changes in warfarin dosing in the 7 days preceding the INR tests before and after the start of NMV/r. The average time from the last INR to start NMV/r was 12.9 ± 8.3 days. Before the start of NMV/r, there were an average of 4.6 ± 2.1 measurements (range, 3-12) over 58.7 ± 17.7 days (range, 8-84). After the start of NMV/r: 2.0 ± 1.4 measurements (range, 0-6) over 23.5 ± 22.2 days (range, 3-97). The first INR measurement following Paxlovid completion was at a mean of 7.6 ± 4.7 days (range, 1-22). Among the 29 patients, only 1 (3.4%) measured INR below 2.0 before initiation of NMV/r, compared with 48% (n = 14) in the 2 weeks following NMV/r therapy (Fisher exact test, P < .001). Comparing INR before NMV/r administration (days −1 to −33) with the first posttreatment INR, the median INR decrease was −0.40 (Wilcoxon signed-rank test, P = .18). In the period immediately following the 5-day NMV/r course (days 5-14), the measured INR was significantly lower than the pre-NMV/r (median INR decrease, −0.50; Wilcoxon signed-rank test, P = .026; Figure 1A). For the 13 patients who had INR measured during the active treatment phase (days 0-4), the median INR was 2.00 (range, 1.90-2.40), which was not statistically different compared with these patients’ pretreatment INR (2.40; IQR, 2.20-2.70; P = .13). To assess the INR trends associated with NMV/r therapy, we compared the pretreatment INR value with the lowest or highest INR measured between days 0 and 14 after initiation of NMV/r. Consistent with an overall trend toward lower INR, the lowest measured INR values were significantly decreased compared with baseline (P = .013; Figure 1B), but there was no statistical difference when comparing peak INR to pre-NMV/r INR values (P = .29).
Comparison of INR measurements before and after NMV/r therapy. (A) Comparison of pre-NMV/r INR values with those measured during NMV/r therapy (n = 13) or between days 5 and 14 (n = 27, Wilcoxon signed-rank test, ∗P = .026). (B) Pre-NMV/r INR values compared with the lowest or highest INR measured between days 0 and 14 (Wilcoxon signed-rank test, ∗P = .013).
Comparison of INR measurements before and after NMV/r therapy. (A) Comparison of pre-NMV/r INR values with those measured during NMV/r therapy (n = 13) or between days 5 and 14 (n = 27, Wilcoxon signed-rank test, ∗P = .026). (B) Pre-NMV/r INR values compared with the lowest or highest INR measured between days 0 and 14 (Wilcoxon signed-rank test, ∗P = .013).
No thrombotic events occurred during the study period after the NMV/r administration. One patient with a prior history of bowel resection with diverting ostomy and a pre-NMV/r INR of 2.7 had an abrupt increase in INR of 20 on day 3 of NMV/r therapy and presented with gastrointestinal bleeding. He required reversal with vitamin K and prothrombin complex concentrate. Although the exact site of absorption for ritonavir is currently unknown, its absorption is impacted when administered with food, and case reports describe that patients with HIV who underwent gastric bypass procedures with therapeutic drug monitoring demonstrated a significant impact on absorption.15 Thus, we speculate that in the case described in this series, the interaction was significantly impacted by the altered gastrointestinal anatomy leading to the supratherapeutic INR and bleeding event. None of the remaining 28 patients experienced a hemorrhagic event.
Acute COVID-19 infection is a recognized hypercoagulable state that is believed to be driven by the acute inflammatory milieu.16,17 Patients hospitalized with COVID-19 were identified even early in the pandemic to have increased rates of thrombosis and bleeding,18 although rates appear to be low in patients with milder symptoms who are managed in the ambulatory setting.19 This coagulopathic state has been characterized by laboratory abnormalities including hyperfibrinoginemia, elevated D-dimer, and to a lesser degree, prolonged prothrombin time.20 The impact of acute SARS-CoV-2 infection on INR value and thrombotic bleeding events for patients on chronic warfarin therapy have not been well characterized. In this case series, it is possible that the changes in INR trends were related to acute infection rather than NMV/r treatment. However, given that no patients had severe infections that required hospitalization and the majority were associated with a trend of reduction in INR values, it is likely that the changes observed were owing to drug effects rather than COVID-19 coagulopathy. Finally, although we only included patients taking warfarin in this study, other oral anticoagulants (including direct oral anticoagulants) could also have similar interactions with NMV/r and should be included in further studies.
Given the ongoing pandemic and the increasing use of antiviral agents, there is a need to characterize the impact of NMV/r on warfarin metabolism and monitoring. To the best of our knowledge, this is the first report describing the clinical outcomes in patients with COVID-19 who received NMV/r during warfarin therapy. We observed that approximately half of all patients on warfarin experienced subtherapeutic INR values immediately following NMV/r. We recognize the limitations inherent to a case-series design and await confirmation with larger data sets. Based on these data, we do not encourage empiric holding doses of warfarin when taking NMV/r and recommend close monitoring of INR, especially in the immediate post-NMV/r period.
Authorship
Contribution: O.M., R.P., M.L., and J.I.Z. wrote the manuscript; M.L., T.L., and M.G. collected the data; and O.M., R.P., L.D., and J.I.Z. performed the data analysis.
Conflict-of-interest disclosure: J.I.Z. reports research funding from Incyte and Quercegen; is a member on the data safety monitoring boards for Sanofi and CSL Behring; provides consultancy for Calyx; and is a member on the advisory board participation with Pfizer/Bristol-Myers Squibb, Portola, Janssen, and Daiichi. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey I. Zwicker, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; e-mail: zwickerj@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Jeffrey I. Zwicker (zwickerj@mskcc.org).
O.M. and R.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal