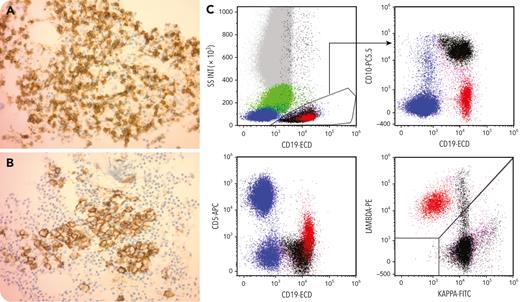
A 75-year-old man with a history of λ-restricted chronic lymphocytic leukemia (CLL), with trisomy 12 and t(14;18), with IGH-BCL2 fusion signals in 65% of cells, developed a λ-restricted plasmacytoma in 2018. He developed multiple myeloma in 2020 with high-risk cytogenetics including TP53 deletion. Bone marrow examination showed a B-cell infiltrate comprising approximately 50% of the marrow cells (panel A, CD20, 40× objective) and a plasma cell infiltrate comprising 10% to 20% of the marrow (panel B, CD138, 40× objective). In 2021, he underwent an autologous stem cell transplant and was placed on daratumumab, bortezomib, and dexamethasone maintenance. In 2022, he underwent a repeat bone marrow examination, which showed residual CLL. Morphologic examination and minimal residual disease flow cytometry did not show evidence of multiple myeloma. Clonality analysis confirmed the presence of one IG λ light-chain gene rearrangement. Flow cytometry (panel C) showed 2 distinct CD19+ populations, namely, a smaller CD20+dim/CD5+ cluster that was light-chain λ-restricted and represented residual CLL (painted red) and a larger κ light chain–expressing CD45dim/SSClow/CD19+/CD10+/CD20− showing typical hematogone maturation (painted black).
Daratumumab is an IgG1 κ monoclonal antibody that binds to CD38 on plasma cells. It also binds to other cells that express CD38, such as hematogones. Flow cytometric analysis of bone marrows from such patients may lead to the detection of a κ-expressing, CD10+ B-cell population, which may be mistaken for a B-cell neoplasm.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal