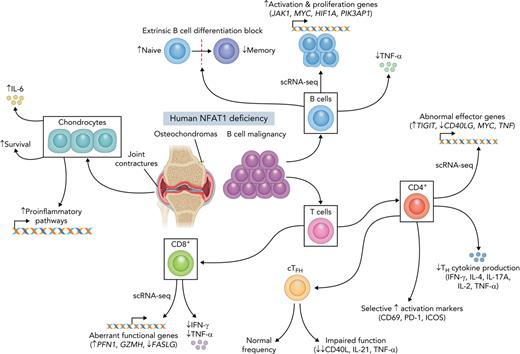
Key Points
Human biallelic damaging variants in NFATC2 increase susceptibility to B-cell lymphoma and musculoskeletal defects.
Studying primary human cell types lacking NFAT1 protein reveals an environment that promotes both cartilage overgrowth and lymphomagenesis.
Abstract
The discovery of humans with monogenic disorders has a rich history of generating new insights into biology. Here we report the first human identified with complete deficiency of nuclear factor of activated T cells 1 (NFAT1). NFAT1, encoded by NFATC2, mediates calcium-calcineurin signals that drive cell activation, proliferation, and survival. The patient is homozygous for a damaging germline NFATC2 variant (c.2023_2026delTACC; p.Tyr675Thrfs∗18) and presented with joint contractures, osteochondromas, and recurrent B-cell lymphoma. Absence of NFAT1 protein in chondrocytes caused enrichment in prosurvival and inflammatory genes. Systematic single-cell–omic analyses in PBMCs revealed an environment that promotes lymphomagenesis with accumulation of naïve B cells (enriched for oncogenic signatures MYC and JAK1), exhausted CD4+ T cells, impaired T follicular helper cells, and aberrant CD8+ T cells. This work highlights the pleiotropic role of human NFAT1, will empower the diagnosis of additional patients with NFAT1 deficiency, and further defines the detrimental effects associated with long-term use of calcineurin inhibitors.
Introduction
Intracellular calcium signaling orchestrates a diverse range of critical physiological and immunological processes, including lymphocyte activation, differentiation, and effector function.1,2 T-cell receptor or B-cell receptor engagement creates an influx of Ca2+ from the endoplasmic reticulum into the cytosol.2 Free Ca2+ is subsequently bound by calmodulin, resulting in a conformation change that promotes binding of the serine/threonine phosphatase calcineurin.3,4 This calmodulin-calcineurin interaction activates an array of transcription factor families, including the nuclear factor of activated T cells (NFAT) family, which consists of 5 members: NFAT1 (NFATc2, NFATp), NFAT2 (NFATc1, NFATc), NFAT3 (NFATc4), NFAT4 (NFATc3, NFATx), and NFAT5.5 Activation of NFAT1-4 is dependent on calcineurin-mediated dephosphorylation of NFATs, exposing a nuclear localization signal, leading to NFAT translocation to the nucleus and subsequent transcriptional regulation.5-12 Originally discovered in activated T cells at the interleukin-2 (IL-2) promoter,13 our understanding of the NFAT family has grown remarkably over the past few decades. It is now appreciated that NFATs have a role in the differentiation and activation of many T helper subsets, including TH1, TH2, TH17, T follicular helper cells (TFH), and T regulatory cells (Tregs).14,15 NFATs are also expressed by and have a function in other immune cells, including B cells16 and dendritic cells,17 as well as in nonimmune cells, such as cartilage cells,18-20 adipocytes,21 cardiac cells,22 and breast cancer cells.23
A particularly illustrative example of how critical calcineurin and NFATs are in immunity and clinical medicine is the fact that calcineurin inhibitors (eg, cyclosporin A, tacrolimus [FK506], and pimecrolimus), which suppress T cells without significant myelotoxicity, have served as the standard of care over the past 40 years for preventing organ rejection and graft-versus-host disease in transplant recipients.10,24-26 Further, they have also been used successfully to treat a variety of immune-mediated disorders, including atopic dermatitis, psoriasis, rheumatoid arthritis, and uveitis.26,27 However, despite the effectiveness of calcineurin inhibitors, serious adverse side effects have been reported, including nephrotoxicity, lymphomas, and increased susceptibility to infections.28 Insight into NFAT biology in humans is thus critical to understand the mechanisms underlying the side effects of these drugs.
One strategy to study human NFAT biology has been to use calcineurin inhibitors.29 However, because they inhibit all members of the NFAT family, the role of individual members has been challenging to define. Similarly, although targeted genetic disruption of individual NFATs in mice has helped uncover their specific functions in murine immunity, this approach is not fully transferable to the human system. A powerful complementary approach is to identify and study individuals who carry damaging germline variants in genes encoding members of the NFAT family, allowing us to investigate their roles in an unmodified human system. Here, we report the identification and characterization of the first human with complete NFAT1 deficiency.
Methods
Study participants and consent
All study participants and/or their parents/guardians provided written informed consent. Research study protocols (H18-02853 and H15-00092) were approved by the University of British Columbia Clinical Research Ethics Board.
Variant identification and Sanger sequencing confirmation
Trio whole-exome sequencing (WES) was performed on genomic DNA from the patient and both parents on an Illumina platform at Ambry Genetics (Aliso Viejo, CA; supplemental Methods, available on the Blood Web site). A predicted null NFATC2 variant (c.2023_2026delTACC, p.Tyr675Thrfs∗18) was selected for further analysis and confirmed by standard Sanger sequencing of genomic DNA from all individuals of the family as previously described30 (details on variant prioritization are provided in supplemental Methods).
Cell isolation, culture, and immortalization of LCLs
Peripheral blood mononuclear cells (PBMCs) were isolated from all study participants by standard Ficoll-Paque (GE Healthcare, Chicago, IL) density centrifugation as previously described30 (supplemental Methods). Lymphoblastoid cell lines (LCLs) were derived by Epstein-Barr virus transformation and cultured in complete RPMI-1640 as previously described30,31 (supplemental Methods).
Chondrocytes were grown from an osteochondroma that was surgically removed from the patient’s proximal tibia. The small cartilage component was excised from connective tissue, digested with 200 U/mL collagenase in complete Dulbecco’s modified Eagle medium (DMEM) (GE Healthcare) for 1 day at 37°C, filtered with a 40-μM cell strainer, and seeded in a monolayer and allowed to adhere.
Stable expression of NFAT1 using a lentivirus vector
Stable expression of NFAT1 was established as previously described.32,33 Briefly, chondrocytes and activated T cells were transduced by GFP-tagged empty vector (EV) or NFATC2 (NM_172091) lentiviral particles and subsequently sorted on green fluorescent protein (GFP) expression using a BD FACS Aria (BD Biosciences) cell sorter (supplemental Methods).
Cytometric bead array
A human inflammatory cytokine bead array (cat. no. 552932; BD Biosciences, Franklin Lakes, NJ) was used to measure IL-6 concentration in the supernatant of stimulated EV- or wild-type (WT)-NFATC2–transduced chondrocytes as previously described34 (supplemental Methods). Samples were acquired using a BD LSRII flow cytometer and analyzed using FlowJo (BD Biosciences).
Immunoblotting
Changes in NFAT1 expression and phosphorylation status were detected in LCLs and chondrocytes. LCLs were stimulated with 10 μM ionomycin for 10 minutes in complete RPMI. EV- or WT-NFATC2–transduced chondrocytes were stimulated in complete DMEM as described in the previous section and supplemental Methods. Protein detection was achieved by standard immunoblotting as previously described30 (supplemental Methods).
Quantification of NFATC2 transcript abundance by quantitative PCR
Quantitative polymerase chain reaction (PCR) was conducted as previously described.30 Briefly, total RNA was extracted from unstimulated LCLs using a RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) and converted to complementary DNA using an iScript complementary DNA synthesis kit (BioRad Laboratories, Hercules, CA). Transcript abundance was measured using Universal SYBR Green Super Mix (Bio-Rad) and a ViiA7 Real-Time PCR System (Thermo Fischer, Waltham, MA. Primers listed in supplemental Methods). Relative transcript abundance was quantified relative to ACTB using the 2−ΔΔCT method.35
RNA sequencing
To investigate the global transcriptome, EV- or WT-NFATC2–transduced chondrocytes were unstimulated or stimulated for 4 hours with phorbol 12-myristate 13-acetate and ionomycin (P/I) or 20 ng/mL IL-1β. RNA was extracted in triplicate, prepared, sequenced, and analyzed according to standard procedures as previously described30 (supplemental Methods). Raw data are deposited in Gene Expression Omnibus with accession number GSE193414.
scRNA-seq
Targeted single-cell RNA sequencing (scRNA-seq) of 500 curated immune-related genes was conducted on unstimulated or 4-hour P/I–stimulated PBMCs from the patient, 3 heterozygous controls, and 2 age-matched healthy controls using the BD Rhapsody Single Cell platform according to the manufacturer’s recommendations. Details on the experimental approach and bioinformatics analyses are included in supplemental Methods. Raw data are deposited in Gene Expression Omnibus with accession number GSE193410. All downstream analysis scripts used in analysis are available on GitHub (https://github.com/maggie-fu/NFATC2_RNAseq/).
LDH assay
Chondrocyte resistance to cell death was quantified using a lactate dehydrogenase (LDH) assay according to the manufacturer’s instructions (cat. no. G1780; Promega, Madison, WI). Briefly, supernatants from EV- or WT-NFATC2–transduced chondrocytes stimulated with P/I over a time course from 0 to 12 days were harvested. LDH release was detected using the CytoTox96 reagent and measured on the Infinite M200 plate reader at 492 nm. Cell death was defined as percentage of maximal LDH release (supplemental Methods).
B-cell expansion and differentiation
Naïve B cells were isolated from PBMCs of the patient, one heterozygous family member, and 6 healthy controls and stimulated with ImmunoCult-ACF Human B Cell Expansion Supplement (cat. no. 10974; Stemcell, Vancouver, Canada; supplemental Methods).
Clinical-grade flow cytometry
Patient clinical immunophenotyping was carried out on whole blood at an accredited clinical flow cytometry laboratory according to standard protocols (supplemental Methods).
Research-grade flow cytometry
To carry out immunophenotyping and intracellular cytokine detection, PBMCs from the patient, heterozygous family members, and age-matched controls were stimulated with P/I for 4 hours at 37°C in the presence of GolgiStop (cat. no. 554724; BD Biosciences). Cells were stained with different antibody panels (listed in supplemental Table 6) using the eBioscience Foxp3 Transcription Factor Staining Buffer Set (cat. no. 00-5523-00; Invitrogen/Thermo Fisher Scientific). Samples were acquired on a BD FACSymphony flow cytometer (BD Biosciences). Data were analyzed using FlowJo (BD Biosciences).
Histology
Formalin-fixed, paraffin-embedded lymph node tissue was sectioned at 4 μM and subjected to routine hematoxylin and eosin staining or immunohistochemistry (supplemental Methods).
Results
Clinical features of complete human NFAT1 deficiency
The patient (designated II-1 on the family pedigree) is a man of Middle Eastern ancestry born to consanguineous parents. Family history was notable for recurrent childhood deaths but with no specific diagnosis. There was no history of neuromuscular disorders or joint contractures, nor childhood malignancies in other family members.
After a healthy birth, at age 1.5 years, the patient started developing difficulty bending his knees with no associated swelling, pain, erythema, or morning stiffness. Insidiously, many joints developed a similar painless decreased range of motion, causing difficulty with ambulation by age of 3 years. Formal orthopedic assessment at age 15 years demonstrated a neurodevelopmentally normal young man with marked bilateral fixed flexion contractures of knees, hips, and ankles (Figure 1A). Multiple upper-limb joints also exhibited reduced range of motion, including wrists, hands, and shoulders (Table 1; supplemental Table 1). Dual-energy X-ray absorptiometry scanning at age 15 years revealed low total body bone mineral density (0.888 g/cm2; z-score for chronologic age, −1.9 [−0.4 after adjusting for height]). Radiographs showed flattening of multiple lower thoracic and lumbar vertebral bodies consistent with vertebral compression fractures. Two osteochondromas/exostoses were also identified: one on the medial aspect of the right proximal tibial metaphysis (2 cm) and a second on the anterior aspect of the right proximal fibula (3 cm; Figure 1B). In summary, the main musculoskeletal findings were painless contractures of the large and small joints of the upper and lower limbs, osteochondromas, and osteopenia (Table 1).
Clinical phenotype of a patient with NFAT1 deficiency, joint contractures, and B-cell malignancy. (A) Radiograph demonstrating the extent to which knees could be straightened, documenting the fixed flexion deformity. (B) Osteochondroma (arrow) on the anterior aspect of the right proximal fibula. (C) Hematoxylin and eosin (H&E) stain and CD20, CD21, CD30, BCL2, and BCL6 immunostain of a patient lymph node biopsy. Scale bars for H&E, CD20, CD30, BCL6, and BCL2 are 50 μm, and that for CD21 is 3 mm. (D) Family pedigree. Half-filled symbols represent heterozygous unaffected individuals, filled symbols represent homozygous affected individual; arrow represent proband; question marks represent ungenotyped. (E) Sanger sequencing of DNA extracted from whole blood of the patient, family members, and a healthy control. Site of 4–base-pair deletion is indicated. (F) Schematic illustrating the protein domains of NFAT1. Location of variant is shown in red. Affected region was aligned to other species. Asterisks indicate full conservation. (G) Schematic illustrating ionomycin-induced activation of calcineurin and NFAT1. (H) NFATC2 transcript abundance relative to β-actin (ACTB) in the patient (II-1), a heterozygous control (II-2), and a healthy control (HC) determined by quantitative PCR. Red diamonds, II-1; blue circles, II-2; green circles, HC. ∗∗P < .01, ∗∗∗∗P< .0001, one-way analysis of variance and Tukey’s post hoc test. (I) Immunoblot of patient (II-1), heterozygous control (II-2), and control-derived LCLs using an N-terminal NFAT1 antibody before and after 10 minutes of ionomycin stimulation with or without FK506 treatment (n = 3).
Clinical phenotype of a patient with NFAT1 deficiency, joint contractures, and B-cell malignancy. (A) Radiograph demonstrating the extent to which knees could be straightened, documenting the fixed flexion deformity. (B) Osteochondroma (arrow) on the anterior aspect of the right proximal fibula. (C) Hematoxylin and eosin (H&E) stain and CD20, CD21, CD30, BCL2, and BCL6 immunostain of a patient lymph node biopsy. Scale bars for H&E, CD20, CD30, BCL6, and BCL2 are 50 μm, and that for CD21 is 3 mm. (D) Family pedigree. Half-filled symbols represent heterozygous unaffected individuals, filled symbols represent homozygous affected individual; arrow represent proband; question marks represent ungenotyped. (E) Sanger sequencing of DNA extracted from whole blood of the patient, family members, and a healthy control. Site of 4–base-pair deletion is indicated. (F) Schematic illustrating the protein domains of NFAT1. Location of variant is shown in red. Affected region was aligned to other species. Asterisks indicate full conservation. (G) Schematic illustrating ionomycin-induced activation of calcineurin and NFAT1. (H) NFATC2 transcript abundance relative to β-actin (ACTB) in the patient (II-1), a heterozygous control (II-2), and a healthy control (HC) determined by quantitative PCR. Red diamonds, II-1; blue circles, II-2; green circles, HC. ∗∗P < .01, ∗∗∗∗P< .0001, one-way analysis of variance and Tukey’s post hoc test. (I) Immunoblot of patient (II-1), heterozygous control (II-2), and control-derived LCLs using an N-terminal NFAT1 antibody before and after 10 minutes of ionomycin stimulation with or without FK506 treatment (n = 3).
Major patient clinical features organized by musculoskeletal or immunological/hematological abnormalities
| Musculoskeletal . | Immunological/hematological . |
|---|---|
| Contractures of upper limb joints, hips, knees, and ankles | Age 18 yr: B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and cHL•Widespread lymphadenopathy: abdominal, inguinal, hepatic, splenic, paratracheal, subcarinal, bone; no bone marrow involvement•Lymph node tissue demonstrates malignant large mono- or binucleated cells within a benign background inflammatory cell infiltrate composed of small lymphocytes, eosinophils, histiocytes, and neutrophils•Lymph node: effaced/abnormal architecture, large atypical CD79a+CD30+CD20+CD15−PAX5+ B cells, positive BCL6 rearrangement, negative BCL2 rearrangement |
| Mobility issues: stiff gait, decreased stride length, and toe walking | |
| Decreased arm swing and knee range of motion. Anterior pelvic tilt with adduction of thighs | |
| Ill-defined sclerotic bands present in the metaphyses of femoral tibia and fibula | |
| Presence of multiple small pedunculated osteochondromas | Age 21 yr: mature B cell lymphoma, subtype: Burkitt lymphoma•Supra- and infradiaphragmatic lymphadenopathy with splenic and marrow involvement•Lymph node: effaced architecture, monomorphic medium-large size cell lymphoid population with prominent nucleoli in chromocenters and CD20+ CD79a+CD10dimKi67+CD30−•Positive for the translocation (8;14)(q24;q32) resulting in IGH-MYC fusion |
| Developed severe spinal cord stenosis at level C3 C4, requiring posterior cervical decompression and fusion | |
| Bone density scan (DEXA):Lumbar spine L1-4: 0.739 (g/cm2); Z-score for chronologic age: −1.4; Z-score corrected for height: +0.2Total left hip: 0.865 (g/cm2); Z-score for chronological age 1.0; Z-score corrected for height: +0.2Total body: 0.888 (g/cm2); Z-score for chronologic age: −1.9; Z-score corrected for height: −0.4 | |
| No evidence of muscle necrosis or regeneration, no increased endomysia fibrous tissue, no inflammation, and no atypical cells | Detectable serum IgG against: cytomegalovirus, EBV capsid, Herpes simplex virus, and Rubella virus |
| Musculoskeletal . | Immunological/hematological . |
|---|---|
| Contractures of upper limb joints, hips, knees, and ankles | Age 18 yr: B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and cHL•Widespread lymphadenopathy: abdominal, inguinal, hepatic, splenic, paratracheal, subcarinal, bone; no bone marrow involvement•Lymph node tissue demonstrates malignant large mono- or binucleated cells within a benign background inflammatory cell infiltrate composed of small lymphocytes, eosinophils, histiocytes, and neutrophils•Lymph node: effaced/abnormal architecture, large atypical CD79a+CD30+CD20+CD15−PAX5+ B cells, positive BCL6 rearrangement, negative BCL2 rearrangement |
| Mobility issues: stiff gait, decreased stride length, and toe walking | |
| Decreased arm swing and knee range of motion. Anterior pelvic tilt with adduction of thighs | |
| Ill-defined sclerotic bands present in the metaphyses of femoral tibia and fibula | |
| Presence of multiple small pedunculated osteochondromas | Age 21 yr: mature B cell lymphoma, subtype: Burkitt lymphoma•Supra- and infradiaphragmatic lymphadenopathy with splenic and marrow involvement•Lymph node: effaced architecture, monomorphic medium-large size cell lymphoid population with prominent nucleoli in chromocenters and CD20+ CD79a+CD10dimKi67+CD30−•Positive for the translocation (8;14)(q24;q32) resulting in IGH-MYC fusion |
| Developed severe spinal cord stenosis at level C3 C4, requiring posterior cervical decompression and fusion | |
| Bone density scan (DEXA):Lumbar spine L1-4: 0.739 (g/cm2); Z-score for chronologic age: −1.4; Z-score corrected for height: +0.2Total left hip: 0.865 (g/cm2); Z-score for chronological age 1.0; Z-score corrected for height: +0.2Total body: 0.888 (g/cm2); Z-score for chronologic age: −1.9; Z-score corrected for height: −0.4 | |
| No evidence of muscle necrosis or regeneration, no increased endomysia fibrous tissue, no inflammation, and no atypical cells | Detectable serum IgG against: cytomegalovirus, EBV capsid, Herpes simplex virus, and Rubella virus |
cHL, classic Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; DEXA, dual-energy X-ray absorptiometry; EBV, Epstein-Barr virus.
At age 18 years, the patient presented with abdominal pain, fever, night sweats, and weight loss (ie, B symptoms) and was ultimately diagnosed with B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classic Hodgkin lymphoma. Lymphoid tissue biopsy showed effacement of typical node architecture and the absence of clear germinal centers and follicular structure. There was a prominent population of larger atypical lymphocytes (CD30+CD20+CD15−BCL6+) in the background of mature CD4+ and CD8+ T cells (Figure 1C). He was staged as Ann Arbor stage 4B with widespread disease, including abdominal, inguinal, hepatic, splenic, paratracheal, and subcarinal lymphadenopathy. The patient was treated with 6 cycles of a modified dose-adjusted regimen of etoposide, prednisone, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R without vincristine) and went into full remission. Unfortunately, 3 years later, at the age of 21 years, the patient presented with axillary adenopathy. Positron emission tomography scanning revealed supra- and infradiaphragmatic lymphadenopathy together with splenic and marrow involvement. Lymph node excisional biopsy revealed effaced architecture caused by a monomorphic medium-large–size cell lymphoid population with prominent nucleoli in chromocenters staining positive for CD20, CD79a, CD10 (dim), and Ki67. Cytogenetics revealed the translocation (8;14)(q24;q32), resulting in an IGH-MYC fusion. A diagnosis of mature B-cell lymphoma, subtype Burkitt lymphoma, was made, and the patient was undergoing treatment at the time of manuscript preparation (Table 1).
WES reveals a novel damaging homozygous variant in NFATC2
Given the unique phenotype of the patient, consanguinity, and significant family history, we performed WES to identify a potential genetic etiology. The patient was found to carry a previously unreported homozygous 4–base-pair deletion in exon 8 of the gene NFATC2 encoding the NFAT1 transcription factor. This variant causes a frame shift leading to a premature stop codon (NM_173091:c.2023_2026delTACC; NP_775114:p.Tyr675Thrfs∗18). Sanger sequencing of the variant region in whole blood confirmed that it segregated with disease within the patient family (Figure 1D-E). The variant localizes to the DNA binding domain of the NFAT1 protein and affects an evolutionarily conserved region (Figure 1F). This variant led to significantly reduced NFATC2 transcript abundance (Figure 1H) and undetectable NFAT1 expression in patient-derived LCLs (Figure 1I). Furthermore, following ionomycin stimulation, we observed increased electrophoretic shift of NFAT1 in the WT and heterozygous control LCLs, likely mediated by NFAT1 dephosphorylation.36 This effect was absent in the patient-derived LCLs and was effectively inhibited by the calcineurin inhibitor tacrolimus, suggesting a functional NFAT1 pathway (Figure 1G,I).
EV-transduced patient chondrocytes are enriched in cell proliferation and inflammatory pathways with increased IL-6 production and resistance to cell death
Informed by the patient’s joint contractures and osteochondromas, we assessed the impact of NFAT1 deficiency on chondrocytes isolated from the excised tibial osteochondroma. Because of the unavailability of a WT control that matched the differentiation state of the patient cells, we constructed and compared EV- and WT NFATC2–transduced patient chondrocytes. EV-transduced (“unrescued”) patient chondrocytes had undetectable NFAT1 protein expression, whereas viral transduction with WT NFATC2 (“rescued” chondrocytes) restored NFAT1 protein expression (Figure 2A). Further, treatment with IL-1β, a key cytokine involved in cartilage damage in arthritis,37 did not change NFAT1 phosphorylation status, whereas treatment with P/I led to an electrophoretic shift of NFAT1 likely mediated by NFAT1 dephosphorylation (Figure 2A).
NFAT1 deficiency in chondrocytes leads to an increased prosurvival and proinflammatory phenotype. (A) Immunoblot of EV- and WT NFATC2–transduced patient chondrocytes before and after 15 minutes of P/I stimulation, 20 ng/mL IL-1β stimulation, or no treatment (n = 3). (B-D) RNA sequencing carried out on EV- or WT NFATC2–transduced patient chondrocytes unstimulated or stimulated for 24 hours with P/I or 20 ng/mL IL-1β. (B) Venn diagram of significantly (FDR < 0.05) upregulated (Bi) and downregulated (Bii) genes in EV-transduced patient chondrocytes compared with WT NFATC2–transduced chondrocytes. (C) Heat map of top genes that meet a FDR < 0.025 cutoff between EV and WT NFATC2–transduced patient chondrocytes. (D) Heat map of sample level enrichment scores with normalized enrichment scores, P-value, and q-values for each pathway determined by gene set enrichment analysis. Min and max refer to the row-normalized minimum and maximum for each pathway. (E) Measurement of percent cell death in chondrocytes over 12 days by quantifying LDH in supernatants. ∗P < .05, ∗∗∗P < .001, two-way analysis of variance, Dunnett’s post-hoc test. (F) Enzyme-linked immunosorbent assay detection of IL-6 production from the supernatants of chondrocytes in different conditions. ∗P < .05, ∗∗∗P < .001, Mann-Whitney U test. NS, not significant.
NFAT1 deficiency in chondrocytes leads to an increased prosurvival and proinflammatory phenotype. (A) Immunoblot of EV- and WT NFATC2–transduced patient chondrocytes before and after 15 minutes of P/I stimulation, 20 ng/mL IL-1β stimulation, or no treatment (n = 3). (B-D) RNA sequencing carried out on EV- or WT NFATC2–transduced patient chondrocytes unstimulated or stimulated for 24 hours with P/I or 20 ng/mL IL-1β. (B) Venn diagram of significantly (FDR < 0.05) upregulated (Bi) and downregulated (Bii) genes in EV-transduced patient chondrocytes compared with WT NFATC2–transduced chondrocytes. (C) Heat map of top genes that meet a FDR < 0.025 cutoff between EV and WT NFATC2–transduced patient chondrocytes. (D) Heat map of sample level enrichment scores with normalized enrichment scores, P-value, and q-values for each pathway determined by gene set enrichment analysis. Min and max refer to the row-normalized minimum and maximum for each pathway. (E) Measurement of percent cell death in chondrocytes over 12 days by quantifying LDH in supernatants. ∗P < .05, ∗∗∗P < .001, two-way analysis of variance, Dunnett’s post-hoc test. (F) Enzyme-linked immunosorbent assay detection of IL-6 production from the supernatants of chondrocytes in different conditions. ∗P < .05, ∗∗∗P < .001, Mann-Whitney U test. NS, not significant.
We next investigated the impact of rescued NFAT1 expression on the global transcriptome of chondrocytes. In the P/I-stimulated condition, we identified 187 significantly upregulated and 146 significantly downregulated genes comparing EV- and WT NFATC2–transduced chondrocytes (Figure 2B-C). Gene set enrichment analysis showed notable enrichment of cell proliferation pathways at baseline and in response to P/I stimulation in EV versus NFATC2-transduced chondrocytes, including G2/M checkpoint (unstimulated: normalized enrichment score [NES] = 1.790, false discovery rate [FDR] < 0.001; P/I-stimulated: NES = 1.544, FDR = 0.047) and E2F targets (unstimulated: NES = 1.704, FDR = 0.004; stimulated: NES = 1.667, FDR = 0.013; Figure 2D). Validating these transcriptomic findings functionally, EV-transduced chondrocytes showed significantly more resistance to cell death than cells with rescued WT NFATC2 (Figure 2E).
The IL-6-JAK-STAT3 signaling pathway was also significantly enriched in unstimulated EV-transduced chondrocytes compared with WT NFATC2–transduced cells (NES = 1.543, FDR = 0.035; Figure 2D). Confirming this at the protein level, unstimulated and P/I-stimulated EV-transduced chondrocytes produced significantly more IL-6 than rescued WT NFATC2–transduced chondrocytes (Figure 2F). Taken together, these findings suggest that NFAT1 regulates chondrocyte growth and promotes death while limiting proinflammatory transcriptional programs, collectively leading to aberrant connective tissue homeostasis in NFAT1 deficiency.
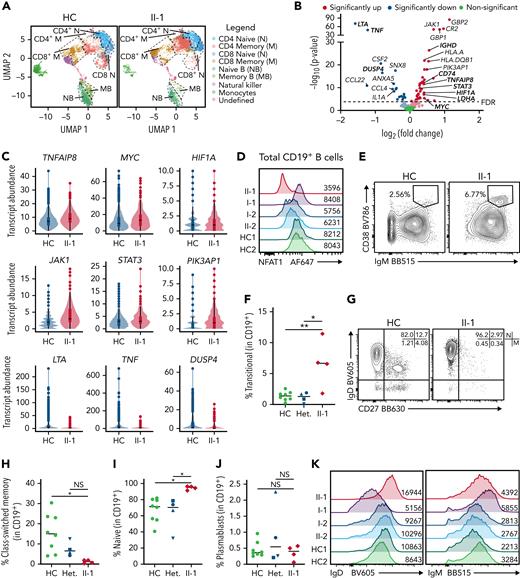
Patient B cells are largely naïve ex vivo and express proliferation markers
Given that NFAT1 is expressed in most lymphocytes,16 we conducted targeted scRNA-seq on the patient’s PBMCs alongside 5 healthy controls to identify cell type–specific defects. Unsupervised clustering analysis revealed markedly reduced memory B cells and elevated memory CD4+ T cells in the patient PBMCs (Figure 3A). Because patient B cells were largely naïve, we conducted differential gene expression (DE) analysis on naïve B cells, and, in the NFAT1-deficient patient cells, we discovered significantly increased transcript abundance of prosurvival, proliferation, and antiapoptotic genes frequently associated with lymphoma (Figure 3B). This includes MYC,38HIF1A,39JAK1, STAT3,40TNFAIP8,41 and PIK3AP142 (Figure 3C). Consistent with the documented NFAT1 deficiency, classical NFAT1 targets such as TNF and LTA43,44 were also significantly decreased.
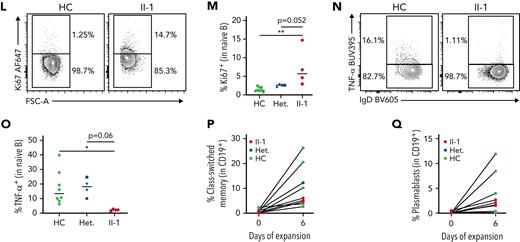
NFAT1-deficient patient has an accumulation of hyperproliferative naïve B cells. (A-C) scRNA-seq of PBMCs from the patient (II-1), heterozygous controls (n = 3), and healthy control (n = 2) stimulated with P/I for 4 hours. (A) Uniform manifold approximation and projection (UMAP) visualization of stimulated cell subsets. Cell labels indicated on right. (B) Volcano plot showing DE in naïve B cells between patient and 5 healthy controls. Dark colors, significance; dim colors, nominal significance but not after adjusted P value; green, not significant. (C) Violin plots of significantly differentially expressed genes of interest in naïve B cells comparing patient and healthy controls. (D) Expression of NFAT1 in patient and control naïve B cells. Mean fluorescence intensities (MFIs) are indicated. (E) Frequency of IgM++CD38++ transitional B cells in the patient (II-1) and heterozygous (n = 4) and healthy controls (n = 8). (F) Quantification of E. (G) Frequency of IgD+CD27− naïve (marked as “N”) and IgD−CD27+ memory (marked as “M”) B cells in the patient and one representative control. Schematic of quadrants that correspond to each cell population and frequency shown at top right. (H-I) Quantification of G. (J) Frequency of CD27+CD38+ plasmablasts in mature CD19+ B cells. (K) IgD and IgM expression in each individual. Mean fluorescence intensities are indicated. (L) Frequency of Ki67+ naïve B cells in the patient and a representative control. (M) Quantification of L. (N) Frequency of TNF-α+ naïve B cells in the patient and a representative control. (O) Quantification of N. (P-Q) Isolated naïve B cells from patient and heterozygous (n = 1) and healthy controls (n = 6) expanded for 6 days. (P) Frequency of class-switched memory B cells and (Q) plasmablasts before and after expansion. ∗P < .05, ∗∗P < .01, one-way analysis of variance and Tukey’s post hoc test. NS, not significant. Green circles, healthy control; blue circle, II-2; blue upright triangle, I-2; blue inverted triangle, I-1; blue square, II-3; red diamond, II-1.
NFAT1-deficient patient has an accumulation of hyperproliferative naïve B cells. (A-C) scRNA-seq of PBMCs from the patient (II-1), heterozygous controls (n = 3), and healthy control (n = 2) stimulated with P/I for 4 hours. (A) Uniform manifold approximation and projection (UMAP) visualization of stimulated cell subsets. Cell labels indicated on right. (B) Volcano plot showing DE in naïve B cells between patient and 5 healthy controls. Dark colors, significance; dim colors, nominal significance but not after adjusted P value; green, not significant. (C) Violin plots of significantly differentially expressed genes of interest in naïve B cells comparing patient and healthy controls. (D) Expression of NFAT1 in patient and control naïve B cells. Mean fluorescence intensities (MFIs) are indicated. (E) Frequency of IgM++CD38++ transitional B cells in the patient (II-1) and heterozygous (n = 4) and healthy controls (n = 8). (F) Quantification of E. (G) Frequency of IgD+CD27− naïve (marked as “N”) and IgD−CD27+ memory (marked as “M”) B cells in the patient and one representative control. Schematic of quadrants that correspond to each cell population and frequency shown at top right. (H-I) Quantification of G. (J) Frequency of CD27+CD38+ plasmablasts in mature CD19+ B cells. (K) IgD and IgM expression in each individual. Mean fluorescence intensities are indicated. (L) Frequency of Ki67+ naïve B cells in the patient and a representative control. (M) Quantification of L. (N) Frequency of TNF-α+ naïve B cells in the patient and a representative control. (O) Quantification of N. (P-Q) Isolated naïve B cells from patient and heterozygous (n = 1) and healthy controls (n = 6) expanded for 6 days. (P) Frequency of class-switched memory B cells and (Q) plasmablasts before and after expansion. ∗P < .05, ∗∗P < .01, one-way analysis of variance and Tukey’s post hoc test. NS, not significant. Green circles, healthy control; blue circle, II-2; blue upright triangle, I-2; blue inverted triangle, I-1; blue square, II-3; red diamond, II-1.
To study this B cell phenotype further, we carried out multiparameter flow cytometric analyses (supplemental Figure 1A shows the gating strategy). Direct detection of NFAT1 protein confirmed the lack of NFAT1 in patient B cells compared with controls (Figure 3D). Validating our scRNA-seq data, and consistent with clinical-grade flow cytometry (supplemental Table 2), patient B cells showed signs of a developmental arrest, with significantly increased transitional B cells (Figure 3E-F) and naïve B cells (Figure 3G,I) with a concurrent reduction in class-switched memory B cells (Figure 3G-H). Interestingly, despite the reduced frequency of memory B cells in the patient, plasmablast numbers were comparable to controls (Figure 3J; supplemental Figure 1A), perhaps explaining the patient’s normal serum immunoglobulin levels together with detectable antibody titers against vaccine antigens and past infections (Table 1).
Expanding our naïve B cell analyses further, we observed markedly increased IgD expression (Figure 3K; supplemental Figure 1B) and generally comparable to control IgM expression (Figure 3K; supplemental Figure 1C) in the patient. These IgDhi naïve B cells were characterized by increased expression of the cell proliferation marker Ki67 (Figure 3L-M), but significantly reduced tumor necrosis factor (TNF)-α expression (Figure 3N-O; supplemental Figure 1D).
Given the striking B cell differentiation defect in the patient, we asked whether this was intrinsic or extrinsic to B cells. Naïve B cells expanded and activated in vitro demonstrated intact B cell class-switching (Figure 3P; supplemental Figure 1E) and plasmablast differentiation (Figure 3P-Q) in the NFAT1-deficient patient, suggesting that at least IgG class-switching is extrinsic to B cells.
Patient CD8+ T cells show impaired polyfunctionality
Because NFAT1 deficiency and calcineurin inhibitor treatment are known to suppress CD8+ T cell effector function,45,46 and suppressed CD8+ T cells can create conditions that favor lymphomagenesis,47 we next analyzed CD8+ T cell differentiation and function by scRNA-seq and flow cytometry. DE analysis revealed a mixed effector functional defect, including decreased transcript abundance of TNF, IFNG, and FASLG, but increased GZMH, GNLY, and PFN1 (Figure 4A-B). Flow cytometric analysis of CD8+ T cell subsets revealed no differences in patient naïve and memory populations (Figure 4C-D) and intact IL-2 expression (supplemental Figure 2A,D). As anticipated, patient CD8 populations were NFAT1-deficient (Figure 4E), similar to B cells and chondrocytes. Additionally, patient CD8+ memory T cells showed significantly impaired expression of TNF-α and interferon (IFN)-γ expression (Figure 4F-H). They also exhibit abnormal expression of activation markers. CD40L expression was reduced (supplemental Figure 2E-F), PD-1 expression was intact (Figure 4I; supplemental Figure 2H), and CD69 expression was increased (Figure 4I; supplemental Figure 2G). These findings indicate a pleiotropic function for NFAT1 in human CD8+ T cells.
NFAT1-deficient patient has impaired memory CD8+T cell function. (A) Volcano plot showing DE in CD8+ memory T cells between patient and 5 healthy controls (3 heterozygous controls and 2 healthy controls). (B) Violin plots of significantly differentially expressed genes of interest in CD8+ memory T cells in patient and healthy controls. (C) Frequency of CD8+ T cell subsets in the patient and a representative control, including CD45RA+CD27− TEMRA, CD45RA+CD27+ naïve (marked as “N”), CD45RA−CD27− effector memory (EM), and CD45RA−CD27+ central memory (CM). Quadrants corresponding to each subset are shown at top right. (D) Quantification of C. (E) Expression of NFAT1 in patient and control naïve and memory CD8+ T cells. Mean fluorescence intensities are indicated. (F) TNF-α and IFN-γ expression in memory CD8+ T cells in the patient and a control. (G-H) Quantification of F. (I) CD69 and PD-1 expression in patient and control memory CD8+ T cells after 4 hours of P/I stimulation. Mean fluorescence intensities are indicated. ∗P < .05, ∗∗∗∗P < .0001, one-way analysis of variance and Tukey’s post-hoc test. Green circles, healthy control; blue circle, II-2; blue upright triangle, I-2; blue inverted triangle, I-1; blue square, II-3; red diamond, II-1.
NFAT1-deficient patient has impaired memory CD8+T cell function. (A) Volcano plot showing DE in CD8+ memory T cells between patient and 5 healthy controls (3 heterozygous controls and 2 healthy controls). (B) Violin plots of significantly differentially expressed genes of interest in CD8+ memory T cells in patient and healthy controls. (C) Frequency of CD8+ T cell subsets in the patient and a representative control, including CD45RA+CD27− TEMRA, CD45RA+CD27+ naïve (marked as “N”), CD45RA−CD27− effector memory (EM), and CD45RA−CD27+ central memory (CM). Quadrants corresponding to each subset are shown at top right. (D) Quantification of C. (E) Expression of NFAT1 in patient and control naïve and memory CD8+ T cells. Mean fluorescence intensities are indicated. (F) TNF-α and IFN-γ expression in memory CD8+ T cells in the patient and a control. (G-H) Quantification of F. (I) CD69 and PD-1 expression in patient and control memory CD8+ T cells after 4 hours of P/I stimulation. Mean fluorescence intensities are indicated. ∗P < .05, ∗∗∗∗P < .0001, one-way analysis of variance and Tukey’s post-hoc test. Green circles, healthy control; blue circle, II-2; blue upright triangle, I-2; blue inverted triangle, I-1; blue square, II-3; red diamond, II-1.
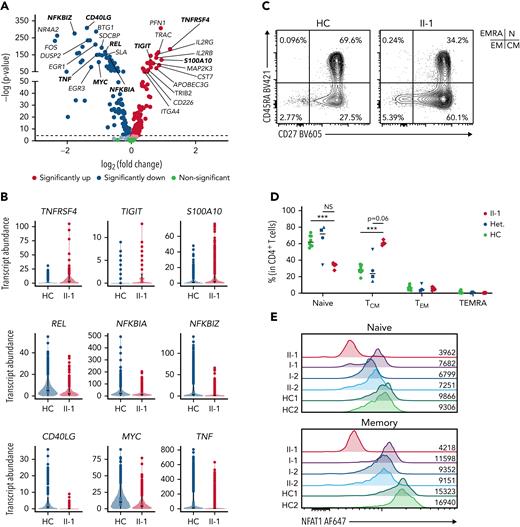
Patient has an accumulation of nonfunctional memory CD4+ T cells
To investigate the role of NFAT1 in CD4 T cell functionality and differentiation in humans, we employed scRNA-seq, which revealed a marked increase in the memory CD4+ T cell compartment (Figure 3A). DE analysis on these cells found significantly impaired expression of makers for T helper function (ie, TNF and CD40LG), impaired expression of regulatory components of the NF-ĸB pathway (NFKBIZ, NFKBIA, REL) and AP-1 pathway (FOS and JUN), as well as increased expression of activation/exhaustion-related genes such as TIGIT, S100A10, and TNFRSF4 (Figure 5A-B).
NFAT1-deficient patient CD4+T cells are exhausted. (A) Volcano plot showing DE in CD4+ memory T cells between patient and 5 healthy controls. (B) Violin plots of significantly differentially expressed genes of interest in CD4+ memory T cells in patient and healthy controls. (C) Frequency of CD4+ T cell subsets in the patient and a representative control, including CD45RA+CD27− TEMRA, CD45RA+CD27+ naïve (marked as “N”), CD45RA−CD27− effector memory (EM), and CD45RA−CD27+ central memory (CM). Quadrants corresponding to each subset are shown at top right. (D) Quantification of C. (E) Expression of NFAT1 in patient and control naïve and memory CD4+ T cells. Mean fluorescence intensities are indicated. (F) Frequency of TH1 (IFN-γ+IL-17A−), TH17 (IL-17A+IFN-γ+) cells in memory CD4+ T cells of the patient and a representative control. (G-H) Quantification of F. (I) Frequency of TH2 (IL-4+IL-21−) and IL21+ (IL-21+IL-4−) cells in memory CD4+ T cells of the patient and a representative control. (J-K) Quantification of I. (L) Frequency of TNF-α+ and IL-2+ CD4+ memory T cells in the patient and a representative control. (M-N) Quantification of L. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, one-way analysis of variance and Tukey’s post-hoc test. NS, not significant. Green circles, healthy control; blue circle, II-2; blue upright triangle, I-2; blue inverted triangle, I-1; blue square, II-3; red diamond, II-1.
NFAT1-deficient patient CD4+T cells are exhausted. (A) Volcano plot showing DE in CD4+ memory T cells between patient and 5 healthy controls. (B) Violin plots of significantly differentially expressed genes of interest in CD4+ memory T cells in patient and healthy controls. (C) Frequency of CD4+ T cell subsets in the patient and a representative control, including CD45RA+CD27− TEMRA, CD45RA+CD27+ naïve (marked as “N”), CD45RA−CD27− effector memory (EM), and CD45RA−CD27+ central memory (CM). Quadrants corresponding to each subset are shown at top right. (D) Quantification of C. (E) Expression of NFAT1 in patient and control naïve and memory CD4+ T cells. Mean fluorescence intensities are indicated. (F) Frequency of TH1 (IFN-γ+IL-17A−), TH17 (IL-17A+IFN-γ+) cells in memory CD4+ T cells of the patient and a representative control. (G-H) Quantification of F. (I) Frequency of TH2 (IL-4+IL-21−) and IL21+ (IL-21+IL-4−) cells in memory CD4+ T cells of the patient and a representative control. (J-K) Quantification of I. (L) Frequency of TNF-α+ and IL-2+ CD4+ memory T cells in the patient and a representative control. (M-N) Quantification of L. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, one-way analysis of variance and Tukey’s post-hoc test. NS, not significant. Green circles, healthy control; blue circle, II-2; blue upright triangle, I-2; blue inverted triangle, I-1; blue square, II-3; red diamond, II-1.
Confirming our scRNA-seq data, flow cytometry showed that the patient possessed decreased naïve CD4+ T cells with a concurrent increase in CD4+ central memory T cells (Figure 5C-D; supplemental Table 2), and, as expected, both these subsets had undetectable levels of NFAT1 protein, in contrast to the controls (Figure 5E). Within the memory CD4+ T cell compartment, the patient had significantly decreased TH1, TH2, TH17, and IL-21+ cells as measured by cytokine (ie, IFN-γ, IL-4, IL-17A, and IL-21) production (Figure 5F-K). Furthermore, classical NFAT1 targets IL-2 and TNF-α7 were significantly reduced as well (Figure 5L-N). In contrast, naïve CD4+ T cells did not exhibit any significant differences in the expression of these same cytokines, but the comparison here was difficult to establish as a result of low cytokine expression levels in healthy control naïve CD4+ T cells (supplemental Figure 3A-I; supplemental Table 3). Human NFAT1 deficiency was also investigated in patient T regulatory cells and showed no differences from controls in frequencies or cytokine production (supplemental Figure 5).
NFAT1 deficiency is associated with impaired TFH function
The expression of activation markers and critical T cell coreceptors by CD4+ memory T cell population was next studied in the context of NFAT1 deficiency. Here we found significantly increased CD69, PD-1, and ICOS, but decreased CD25 and CD40L, after P/I treatment (Figure 6A; supplemental Figure 3P-T). A similar profile was observed in naïve CD4+ T cells (supplemental Figures 3K-5O).
NFAT1 deficiency is associated with impaired TFHfunction. (A) Expression of activation markers in CD4+ memory T cells after P/I treatment, including CD69, PD-1, CD40L, CD25, and ICOS. Mean fluorescence intensities are indicated. (B) Frequency of circulating TFH cells in PD-1+ memory CD4+ T cells in the patient and a representative control. (C) Quantification of B. (D) CD40L and IL-21 expression in patient and control cTFH cells after 4 hours of P/I stimulation. (E-F) Quantification of D. (G) Frequency of CD40L+ and TNF-α+ GFP+ CD4+ patient or control memory T cells after 4 hours of P/I stimulation of EV or WT NFATC2 transduction. (H-I) Quantification of G. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, one-way analysis of variance and Tukey’s post-hoc test. NS, not significant. Green circles, healthy control; blue circle, II-2; blue upright triangle, I-2; blue inverted triangle, I-1; blue square, II-3; red/purple diamond, II-1.
NFAT1 deficiency is associated with impaired TFHfunction. (A) Expression of activation markers in CD4+ memory T cells after P/I treatment, including CD69, PD-1, CD40L, CD25, and ICOS. Mean fluorescence intensities are indicated. (B) Frequency of circulating TFH cells in PD-1+ memory CD4+ T cells in the patient and a representative control. (C) Quantification of B. (D) CD40L and IL-21 expression in patient and control cTFH cells after 4 hours of P/I stimulation. (E-F) Quantification of D. (G) Frequency of CD40L+ and TNF-α+ GFP+ CD4+ patient or control memory T cells after 4 hours of P/I stimulation of EV or WT NFATC2 transduction. (H-I) Quantification of G. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, one-way analysis of variance and Tukey’s post-hoc test. NS, not significant. Green circles, healthy control; blue circle, II-2; blue upright triangle, I-2; blue inverted triangle, I-1; blue square, II-3; red/purple diamond, II-1.
Reduced CD4+ memory T cell activation marker expression and cytokine secretion paired with an extrinsic B-cell class-switching defect prompted us to assess the frequency and function of circulating TFH cells in the patient (supplemental Figure 4A). In contrast to IL-21 cytokine staining in CD4+ memory cells (Figure 5F-K), TFH numbers were comparable to controls (Figure 6B-C). The expression of CD40L and IL-21, both important for B cell activation and differentiation,48 was markedly impaired (Figure 6D-F). Importantly, these defects were reversed when we transduced patient CD4+ T cells with WT NFATC2. CD40L and TNF-α expression (IL-21 was not studied because of sample limitations) were restored to a level that was comparable to healthy controls (Figure 6G-I).
Discussion
Here we present a comprehensive clinical, immunological, biochemical, and transcriptional workup of the first reported case of human complete NFAT1 deficiency in a young man with a clinical triad of progressive joint contractures, osteochondromas, and B-cell malignancy. Based on our study, patients should be worked up for possible NFAT1 deficiency if they present with this triad of features. Causality for the variant was established following guidelines proposed by Casanova and colleagues for genetic studies in single patients with immune defects.49 Specifically, we demonstrated that (i) the NFATC2 genotype is monogenic and that the phenotype is rare, distinctive, and occurs with complete penetrance; (ii) expression and function of NFAT1, the gene product NFATC2, were significantly impaired; and (iii) specific defects could be rescued through the reexpression of WT NFAT1. Further support for causality comes from the fact that the musculoskeletal and immunological abnormalities observed in the patient closely recapitulate the phenotype of NFAT1-knockout mice (as fully outlined in Table 2). Although the full phenotype of human NFAT1 deficiency will only be appreciated when more affected individuals are identified, other clinical flags include (i) impaired B-cell development (increased naïve, decreased memory), (ii) normal CD8+ T cell development but impaired TNF-α and IFN-γ production and increased CD69 expression, and (iii) increased CD4+ memory proportions with reduced TH subsets and cytokine secretion and increased CD69, PD-1, and ICOS expression. Notably, haploinsufficiency was not observed, as all heterozygous family members were healthy and unaffected.
Comparison of human NFAT1 deficiency, Nfatc2−/− mice, and patients receiving calcineurin inhibitors
| Feature . | NFAT1-deficient patient . | Nfatc2−/− knockout mice . | Use of calcineurin inhibitors . |
|---|---|---|---|
| Skeletal abnormalities and cartilage/chondrocyte defects | Joint contractures, difficulty in ambulation. Decreased range of motion in joints | Joint contractures, difficulty in ambulation; decreased range of motion in joints18 | – |
| Increased cell cycling, resistance to apoptosis in osteochondroma chondrocytes | Invasive cartilage cells in vivo and contact-induced growth inhibition in vitro, chondrosarcoma18 | Proliferation of chondrocytes from articular cartilage69 | |
| Increased MMP9 and increased IL6/IL-6-STAT3 pathway in patient chondrocytes | Susceptible to osteoarthritis, increased catabolic and decreased anabolic activity in articular cartilage, increased IL620 | – | |
| Low total body bone mineral density with vertebral compression fractures | Decreased bone volume19 | Posttransplant osteoporosis in organ recipients,55 reduced bone mass and osteoporosis in FK506-treated mice in mice19 and rats70 | |
| B cell lymphoma and malignancies | B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and cHL at age 18 y; Burkitt lymphoma at age 21 y | Effaced lymph node architecture, increased incidence of B cell malignancies, features of anaplastic and/or plasmablastic plasmacytomas, and none in Nfatc2+/−53 | Posttransplant lymphoproliferative disorder mostly in EBV+ B cells,71,72 anti-apoptotic effects in Burkitt’s lymphoma cell line,73 increased incidence of lymphoma with topical calcineurin inhibitors74,75 |
| CD4+ T cell impairments | Higher activated (CD69+) T cells, accumulation of CD4+ central memory that exhibit high PD-1 expression | Age-related increase in accumulation of activated CD69+ T cells and memory T cells in secondary lymphoid organs53 | Increase in CD4+ T cell exhaustion post-kidney and post-liver transplants but not CD8+76-78 |
| T follicular helper cell impairment | TFH cells activation is impaired and there is a lack of B cell class switching | Nfatc1/Nfatc2 double knockout mice: impaired TFH differentiation and function, impaired GC formation15 | Decreased TFH numbers and function, low TFH-mediated B cell differentiation in transplant patients79,80 |
| Feature . | NFAT1-deficient patient . | Nfatc2−/− knockout mice . | Use of calcineurin inhibitors . |
|---|---|---|---|
| Skeletal abnormalities and cartilage/chondrocyte defects | Joint contractures, difficulty in ambulation. Decreased range of motion in joints | Joint contractures, difficulty in ambulation; decreased range of motion in joints18 | – |
| Increased cell cycling, resistance to apoptosis in osteochondroma chondrocytes | Invasive cartilage cells in vivo and contact-induced growth inhibition in vitro, chondrosarcoma18 | Proliferation of chondrocytes from articular cartilage69 | |
| Increased MMP9 and increased IL6/IL-6-STAT3 pathway in patient chondrocytes | Susceptible to osteoarthritis, increased catabolic and decreased anabolic activity in articular cartilage, increased IL620 | – | |
| Low total body bone mineral density with vertebral compression fractures | Decreased bone volume19 | Posttransplant osteoporosis in organ recipients,55 reduced bone mass and osteoporosis in FK506-treated mice in mice19 and rats70 | |
| B cell lymphoma and malignancies | B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and cHL at age 18 y; Burkitt lymphoma at age 21 y | Effaced lymph node architecture, increased incidence of B cell malignancies, features of anaplastic and/or plasmablastic plasmacytomas, and none in Nfatc2+/−53 | Posttransplant lymphoproliferative disorder mostly in EBV+ B cells,71,72 anti-apoptotic effects in Burkitt’s lymphoma cell line,73 increased incidence of lymphoma with topical calcineurin inhibitors74,75 |
| CD4+ T cell impairments | Higher activated (CD69+) T cells, accumulation of CD4+ central memory that exhibit high PD-1 expression | Age-related increase in accumulation of activated CD69+ T cells and memory T cells in secondary lymphoid organs53 | Increase in CD4+ T cell exhaustion post-kidney and post-liver transplants but not CD8+76-78 |
| T follicular helper cell impairment | TFH cells activation is impaired and there is a lack of B cell class switching | Nfatc1/Nfatc2 double knockout mice: impaired TFH differentiation and function, impaired GC formation15 | Decreased TFH numbers and function, low TFH-mediated B cell differentiation in transplant patients79,80 |
cHL, classic Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus.
Studying Nfatc2−/− mice has been extremely informative in defining the physiological functions of NFAT1. This includes regulation of chondrogenesis18 and bone development,19,50 maintenance of steady-state hematopoiesis,50 suppression and regulation of T cells,51,52 and B-cell lymphomagenesis.53 Despite reported differences between human and mouse splicing in NFATC2,54 human NFAT1 deficiency recapitulates a selection of the features seen in the knockout mice (Table 2). Although we do not see enhanced immune responses51,52 or dysfunction in hematopoiesis,50 the joint contractures and progressive difficulty in ambulation observed in our NFAT1-deficient patient were accurately predicted by Nfatc2−/− mice, as were the excess differentiation, proliferation, and endochondral ossification affecting cartilage cells.18 The progrowth, prosurvival, and proinflammatory phenotype of patient chondrocytes suggests that human NFAT1 serves as a repressor of cartilage cell growth and differentiation. Interestingly, similar to our patient, bone fractures and osteopenia have also been observed in Nfatc2−/− or tacrolimus-treated mice19 and in organ transplant recipients receiving immunosuppressive regimens that include tacrolimus,55 further strengthening the link to NFAT1 function. In light of these observations, it is important to consider other findings in Nfatc2−/− mice, notably the development of chondrosarcomas18 and susceptibility to osteoarthiritis,20 and we suggest that patients with NFAT1 deficiency should be screened for these conditions.
One of the most significant phenotypes in our patient was the development of a rare mature B cell lymphoma characterized by large CD20+CD30+CD15−BCL6+ cells, followed by the development of Burkitt lymphoma 3 years later. The diagnosis of a second lymphoma suggests the presence of a niche that promotes lymphomagenesis in the patient. Our findings, along with previous literature, strongly implicate NFAT1 as having a role in driving this lymphomagenesis. An increased incidence of anaplastic and/or plasmablastic plasmacytomas—also rare—was previously observed with aging Nfatc2−/− mice.53 Abrogation of NFAT1 function in B cells confers an intrinsic prosurvival program through cyclin E (CCNE2).56 Our scRNA-seq data further suggest that JNK (via DUSP457), AKT (via PIK3AP142), and JAK/STAT signaling (via JAK1 and STAT3) in B cells is contributing to this process. In parallel, the CD4+ T cells of the NFAT1-deficient patient expressed high levels of the inhibitory checkpoint marker PD-1, which has frequently been associated with B-cell lymphomas58,59 and could inhibit effective antitumor effector functions in the patient. This may be further exacerbated by the markedly reduced production of effector cytokines (TNF-α, IFN-γ) in the patient’s CD4+ and CD8+ T cells. The exact mechanism driving lymphomagenesis will need to be elucidated in future studies. This could involve the use of CRISPR to delete NFAT1 in primary healthy control lymphocytes and comparing them to patient cells to delineate NFAT1 targets from those dependent on cell differentiation state, generating patient-derived and gene-edited pluripotent stem cells to investigate lymphocyte differentiation and functions, or transplant of NFAT1-deficient patient and WT-NFATC2 rescued immortalized patient cells into immunodeficient mice to further investigate lymphoma development and explore potential therapeutic options. These findings could indeed guide future targeted therapies, eg, tocilizumab for IL-6 blockade.60 However, based on current evidence, we believe adherence to conventional treatment strategies, ongoing clinical tumor surveillance, and a heightened awareness of tumor risk will be important aspects of the long-term care of patients with NFAT1 deficiency.
Studying complete human germline NFAT1 deficiency can also provide new insights into the use of calcineurin inhibitors and improves our understanding of the consequences of long-term NFAT1 inhibition by calcineurin inhibitors (Table 2). Patients undergoing solid organ or allogeneic hematopoietic stem cell transplantation are frequently treated with an immunosuppressive regimen that includes a calcineurin inhibitor for extended periods of time. This can lead to a rare complication called posttransplant lymphoproliferative disorder (PTLD). Most PTLDs are B cell proliferative disorders believed to result from impaired CD8+ T cell function and Epstein-Barr virus infection.61,62 Our findings complement this understanding, as NFAT1-deficient CD8+ T cells express significantly reduced cytotoxic mediators such as FASLG and IFNG. Consistent with NFAT1-dependent regulation of the transcription factor Egr3 being critical for FasL induction,63 we found that EGR3 and FASLG were downregulated in patient memory T cells. Given these findings, future work should focus on NFAT1 inhibition and B cell proliferation/CD4+ T cell exhaustion in the context of PTLD pathophysiology.
Mechanistically, human NFAT1 deficiency has revealed the remarkable pleiotropic and cell-type–specific functions of NFAT1. Informed by this patient, NFAT1 serves as a tumor suppressor in chondrocytes and B cells. NFAT1 is also required for CD4+ T cells to provide the help needed for B-cell class-switching. We find that human NFAT1 is likely dispensable for TFH differentiation but is critical for TFH activation and function. NFAT1 deficiency could also lead to functional exhaustion of CD4+ memory T cells, as supported by high PD-1 and TIGIT expression64 and low cytokine production. Although suppressed cytokine signaling was observed in memory CD4+ T cells, it was not present in patient naïve CD4+ T cells. However, naïve cells displayed signatures of higher activation, with higher PD-1 and CD69, as well as higher expression of T cell activation markers CXCR3,65ITGA4 (an α-subunit of integrin receptors), and IL2RB (CD122), a cytokine receptor recently found to be involved in homeostatic proliferation of naïve CD4+ T cells.66 Higher activation status in naïve T cells might explain the age-associated accumulation of memory T cells observed in knockout mice53,67 and the NFAT1-deficient patient.
Given the significant T and B cell dysregulation identified in this NFAT1-deficient patient and the absence of classical features for immunodeficiency (recurrent/severe/unusual infections), we propose to classify human NFAT1 deficiency as a primary immune regulatory disorder.68 Studying this first reported patient has given us unique insight into the role of NFAT1 in the human musculoskeletal and immune systems. Our findings corroborate decades of Nfatc2−/− murine studies while also providing a mechanistic understanding of human NFAT1 deficiency in different cell types. Beyond individual patients, this study also informs the clinical implementation of calcineurin inhibitors and highlights potential adverse consequences of long-term use.
Acknowledgments
We thank the patient and his family for supporting the study. We acknowledge the extended clinical care team for supporting this patient, including the Rare Disease Discovery Hub at BC Children’s Hospital and for their support. We acknowledge Navdeep Sangha at Thomas Jefferson University Hospital for providing his expertise in B-cell lymphomas, the histology laboratories at BC Children’s Hospital and BC Cancer for their assistance in histological and immunohistochemistry, and the Biomedical Research Centre Sequencing Core for their assistance with RNA sequencing and processing.
This work was supported by grants from the Canadian Institutes of Health Research (PJT 178054) (S.E.T.), Genome British Columbia (SIP007) (S.E.T.), and BC Children’s Hospital Foundation. S.E.T. holds a Tier 1 Canada Research Chair in Pediatric Precision Health and is the Aubrey J. Tingle Professor of Pediatric Immunology. M.S. is supported by a CIHR Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award (CGS-D) and University of British Columbia Four Year Doctoral Fellowship (4YF). H.Y.L. is supported by a CGS-D, 4YF, Killam Doctoral Scholarship, Friedman Award for Scholars in Health, and a BC Children’s Hospital Research Institute Graduate Studentship. Investigators in the CAUSES Study include: Shelin Adam, Nick Dragojlovic, Christèle du Souich, Alison M. Elliott, Anna Lehman, Larry Lynd, Jill Mwenifumbo, Tanya N. Nelson, Clara van Karnebeek, and Jan M. Friedman.
Authorship
Contribution: M.S., A. Salman, K.L.D.B., M.W., S.C., F.K., M.S.P., and S.E.T. enrolled patients and analyzed clinical data; B.P.M., W.W.W., J.M., A.L., M.S.P. conducted genetic screening and identified variants of significance; M.S., M.P.F., and A.A.S. performed bioinformatic and statistical analysis; M.S., H.Y.L., C.M., S.L., and J.D. performed the laboratory experiments; A. Setiadi performed clinical flow; A.F.L. provided lymph node biopsy of the patient; J.T. and A.C. managed musculoskeletal issues of the patient and extraction of the osteochondroma; and M.S., M.P.F., H.Y.L., A.A.S., C.M., P.M.L., M.S.K., and S.E.T. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stuart E. Turvey, Department of Pediatrics, BC Children’s Hospital, 950 W 28th Ave, Vancouver, BC V5Z 4H4, Canada; e-mail: sturvey@cw.bc.ca.
References
Author notes
∗M.P.F and H.Y.L contributed equally to this study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal