In this issue of Blood, Tirtakusuma et al1 describe aberrations in chromatin modifiers and transcriptional rewiring as drivers of lineage switching in KMT2A-rearranged leukemia.
Shapeshifters, sceadugengan, shadow walkers: these are characters ubiquitous in mythology that can physically transform, from human to beast, for example, to trick, frustrate, and elude their adversaries. Examples are Loki in Norse mythology, Grendel in Beowulf, and Odin and Alberich in the Nibelungenlied. So it is with KMT2A-rearranged (KMT2A-r) leukemia. Also known as MLL (mixed-lineage or myeloid-lymphoid leukemia), rearrangement of KMT2A, encoding the lysine methyltransferase 2A, to a highly diverse range of partner genes, characterizes a group of high-risk acute leukemias of lymphoid, myeloid, or mixed-cell lineage.2,3 These rearrangements, most commonly KMT2A::AFF1 (MLL::AF4), are prevalent (70% to 80%) in infant acute lymphoblastic leukemia (ALL) and are typically associated with a poor prognosis. Disease progression is often accompanied by a drift or switch in lineage, such as from lymphoid to myeloid.4 Loss of CD19 expression that accompanies this shift can result in evasion of CD19-directed immunotherapies, such as blinatumomab and CD19 CAR T-cell therapy.5 Proposed mechanisms for the lineage shift include emergence of a de novo unrelated leukemia, a therapy-mediated selection of primitive pluripotent progenitors that can differentiate toward an alternate lineage, or epigenetic and transcriptomic reprogramming of lineage-committed blasts.6
To explore the molecular basis of lineage promiscuity and shift, Tirtakusuma et al examined 12 cases of MLL::AF4 ALL (6 infant, 2 pediatric, and 2 adult) that relapsed with acute myeloid leukemia (AML) and 2 infants with MLL::AF4 mixed-phenotype acute leukemia (MPAL). Analysis of gene expression data from matched lymphoid presentations and myeloid relapses showed that the lineage switch was accompanied by diminished expression of lymphoid transcription factors (TFs), surface marker genes (eg, PAX5, EBF1, CD19, CD20, and CD22), and immunoglobulin genes, and upregulation of myeloid genes, including CSF3R and members of the CEBP TF family. Analysis of chromatin accessibility showed loss of occupancy of consensus binding sites for lymphoid TFs and vice versa, an increased occupancy of binding motifs for myeloid factors, consistent with the notion that lymphoid-myeloid transition is associated with impairment of chromatin accessibility and occupancy of TF binding sites.
In a subset of cases, the lineage switch was accompanied by mutation and splicing alterations of epigenetic regulators. Among those, chromodomain helicase DNA-binding 4 (CHD4), encoding the ATPase/helicase subunit of the histone-modifying nucleosome remodeling and deacetylase complex, was downregulated in AML relapses compared with matched diagnosis samples of lymphoid lineage, and was differentially spliced in patients with MPAL with the expression of putative loss-of-function isoforms. CHD4 is essential for growth of AML but not for normal hematopoietic cells,7 suggesting that gene dosage is finely regulated.
To demonstrate the role of CHD4 impairment in promoting lineage switching, knockout of CHD4 alone or in combination with other mutated epigenetic modifiers was performed in MLL::AF4-positive ALL cell models, including leukemic cell lines and human cord blood. Knockdown of either CHD4 or PHF3 increased CD33 expression and promoted a transcriptomic profile similar to that of human disease, but only in the context of MLL::AF4, suggesting that lineage switching is promoted by disrupted epigenetic regulation in MLL::AF4 leukemia. Mutational analysis of sorted hematopoietic populations for MLL::AF4 and mutated CHD4 and PHF3 showed that both mutations in CHD4 and PHF3 were unique to the relapse sample and their presence within the purified multipotent progenitor (MPP)–like population suggests that the myeloid relapse most likely originates from a very immature progenitor population, at least in one case.
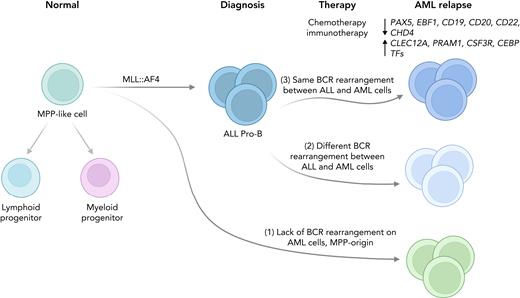
To further explore the cellular origin of lineage-switched relapses, B-cell receptor (BCR) rearrangements were analyzed in the lymphoid lineage samples obtained at presentation and in the matched myeloid lineage samples obtained at relapse. Myeloid relapses shared oncogene fusion breakpoints with their matched lymphoid presentations and were postulated to have originated from different stages of differentiation according to 3 possible scenarios (see figure): (1) the myeloid relapse-initiating cell resides in a primitive precursor (MPP-like) population before early immunoglobulin recombination; (2) myeloid relapse originates from either a myeloid cell with aberrant rearrangement or from a B-lymphoid cell committed to undergoing rearrangement or from a transdifferentiated minor ALL clone with an alternative BCR rearrangement; or (3) myeloid relapse arises from the major ALL clone and shares the same BCR rearrangement.
Models of the cell of origin of lineage-switched relapse in infant KMT2A-r leukemia. Tirtakusuma et al. identified 3 different scenarios: (1) relapsed AML cells lack BCR rearrangement; (2) both ALL and AML cells present BCR rearrangements but they are different; and (3) ALL and AML cells share the same BCR rearrangement.
Models of the cell of origin of lineage-switched relapse in infant KMT2A-r leukemia. Tirtakusuma et al. identified 3 different scenarios: (1) relapsed AML cells lack BCR rearrangement; (2) both ALL and AML cells present BCR rearrangements but they are different; and (3) ALL and AML cells share the same BCR rearrangement.
Recent single-cell sequencing studies of KMT2A-r leukemias supported the hypothesis of a primitive hematopoietic stem/progenitor cell as the cell of origin.8,9 Comparison of single-cell RNA-sequencing (RNA-seq) data of normal fetal bone marrow cells to bulk RNA-seq from childhood ALL and AML showed that infant KMT2A-r ALL exhibits a gene expression profile similar to early lymphocyte precursors (an ELP-like signature), which are oligopotent ELPs that retain minimal myeloid differentiation capacity in vitro. Irrespective of the cellular origin of the relapse, changes in epigenetic networks play a major role, as demonstrated by a recent single-cell multiomic assay for transposase-accessible chromatin using sequencing (ATAC-seq) and RNA-seq of KMT2A-r leukemias and normal hematopoietic cells.8 Single-cell multiomic analyses identified blasts with myeloid potential preceding the lineage switch, which expanded under immunotherapy. Interestingly, the myeloid potential detected by single-cell ATAC-seq exceeded the expression of myeloid signature genes detected by single-cell RNA-seq, suggesting a greater power of epigenomic profiling in detecting lineage potential compared with gene expression changes.8
Collectively, these results build on those of prior studies showing the importance of the intersection of founding genomic alteration and cell of origin as a determinant of lineage ambiguity10 and expand on this finding by demonstrating that changes in chromatin state, which may be determined by secondary mutations in chromatin regulators, may drive lineage shifting under selective pressure. Given the increasing use of leukemic cell antigen-directed immunotherapy, exploiting these mechanistic insights is crucial in anticipating and avoiding lineage shifting and in maximizing the effectiveness of these therapies, ultimately ensuring that these shapeshifting leukemias, like Grendel, are defeated.
Conflict-of-interest disclosure: I.I. has received honoraria from Amgen and Mission Bio. C.G.M. has received research funding from Loxo Oncology, Pfizer, and AbbVie and honoraria from Pfizer, Illumina, and Amgen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal