In this issue of Blood, Li et al1 use different mouse models to comprehensively dissect the role of sphingosine kinases (Sphks) in the regulation of hematopoietic stem cell (HSC) function, uncovering that specific loss of Sphk2 expands and functionally rejuvenates HSCs.
HSCs sustain the lifelong production of most adult blood and immune cell lineages. At the apex of the hematopoietic system, HSCs are characterized by their capacity for long-term self-renewal and their ability to differentiate into mature cells.2 The decline in their regenerative potential is a hallmark of aging, which contributes to the progressive physiological dysfunction and increased risk for age-dependent disorders.3
Multiple studies have tried to elucidate potential mechanisms of HSC aging. In this context, heterochronic parabiosis (a rejuvenating intervention where circulatory systems of aged and young mice are surgically connected) can partially reverse some of these phenotypes. However, whether HSCs are responsive/refractory to this approach remains controversial.4,5 Moreover, the detailed mechanisms mediating its effects are not fully elucidated. Thus, understanding how/why HSCs age is critical to design interventions to prevent, delay, alleviate, or reverse the physiological consequences of aging.
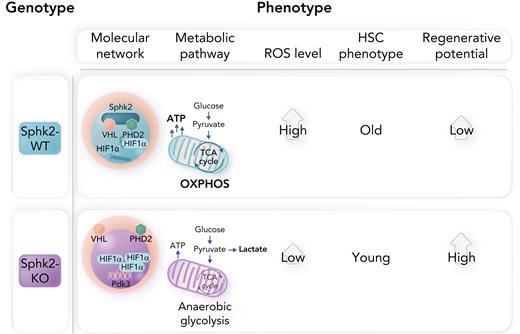
Sphks exist as 2 different isoforms in mammals (Sphk1 and Sphk2), which synthesize sphingosine 1-phosphate (S1P), a bioactive lipid molecule that regulates multiple processes.6 Genetic abrogation of both kinases is embryonically lethal; however, Sphk1-knockout (KO) and Sphk2-KO mice are viable and healthy, suggesting functional redundancy between both.6 In this study, Li and colleagues used a variety of in vitro and in vivo experimental assays to uncover that loss of Sphk2 in the hematopoietic compartment (but not Sphk1) results in expansion of quiescent HSCs without affecting other mature blood cell lineages. Interestingly, loss of Sphk2 is not compensated by increased Sphk1 levels. In addition, even if Sphk2 loss results in decreased S1P levels, the Spkh2-KO phenotype is S1P independent, suggesting that Sphk2 catalytic activity is dispensable for these effects.
To assess the long-term functional relevance of Sphk2 deficiency, the authors performed a series of rigorous reconstitution experiments in vivo. Their results demonstrated that Sphk2-KO bone marrow cells have enhanced self-renewal potential and increased reconstitution capabilities in a cell-autonomous manner, without compromising linage commitment. Even in conditions that force HSC proliferation and differentiation, such as 5-fluorouracil treatment or lethal irradiation, Sphk2-KO mice show significant survival extension, and Sphk2-KO HSCs preserve an improved regenerative potential. This is highly relevant because hematological toxicity is one of the main causes for chemotherapy discontinuation in cancer patients. Thus, small molecules pharmacologically targeting Sphk2 might help prevent/treat these adverse events.
The authors further investigated the role of Sphk2 in HSC aging by integrating computational approaches with additional reconstitution assays comparing HSCs from aged and young mice. Consistent with previous results, Sphk2 deficiency prevents the acquisition of age-induced transcriptional signatures in old HSCs, such that Sphk2-KO old HSCs present phenotypic and functional characteristics like those observed in young wild-type HSCs. In line with this, old HSCs show higher Sphk2 levels than young HSCs. Functional annotation of genes downregulated in Sphk2-KO HSCs suggested reduced oxidative phosphorylation (OXPHOS). Conversely, upregulated genes were involved in the regulation of anaerobic glycolysis. In this context, HSCs are extremely sensitive to reactive oxygen species (ROS) levels.7 Indeed, Sphk2-KO HSCs show increased lactate dehydrogenase activity (and lactate production) and present lower oxygen consumption rates ex vivo, with reductions in both adenosine triphosphate and ROS levels (see figure). It is well known that, in hypoxic environments, quiescent HSCs have low energy demands and mainly rely on anaerobic glycolysis; however, upon activation, they rapidly switch to OXPHOS to satisfy the metabolic requirements of differentiation.7 Li et al’s findings suggest that Sphk2 loss in HSCs might interfere with this metabolic shift, allowing a younger metabolic state that preserves stemness and enhances their regenerative capacity. Interestingly, Sphk2-KO HSCs showed decreased glucose uptake even if increased lactate levels suggest a more glycolytic phenotype. Thus, metabolic tracing with 13C-glucose to experimentally confirm and expand on these apparently conflictive findings would be needed. Current technical limitations prevent from easily using this technique in HSCs; however, recent advances in single-cell and imaging metabolomic approaches could help address this issue in the short-term. Moreover, HSCs show higher Sphk2 levels compared with differentiated cells, suggesting that HSCs need to be metabolically primed to rapidly respond to activation signals and/or that maintenance of the tricarboxylic acid (TCA) cycle in anaerobic conditions is required to provide essential intermediates for the epigenetic regulation of stemness.
Finally, the authors identified HIF1α as a downstream target of Sphk2, consistent with the well-known effects of HIF1α in HSCs.8 Robust in vitro experiments in combination with computational modeling demonstrated that Sphk2 physically interacts with PHD2 and VHL in the HSC nucleus to regulate HIF1α stability in a proteasome-dependent manner (again independently from Sphk2 catalytic activity). Indeed, loss of VHL in hematopoietic progenitors significantly reduced their mitochondrial content,9 suggesting a similar glycolytic phenotype to the one in Sphk2-KO cells.
HIF1α is a key activator of glycolysis in resting HSCs, where it senses low oxygen levels and activates PDK2/PDK4 to prevent pyruvate entry into the TCA cycle by inhibiting pyruvate dehydrogenase (PDH).9 In the context of Sphk2 loss, there is selective upregulation of PDK3 that leads to strongly decreased PDH activity (see figure). Importantly, genetic reduction of either Hif1α or Pdk3 partially rescues the effects of Sphk2 loss by promoting an OXPHOS switch.
In hypoxic environments, loss of Sphk2 increases PDK3 expression via HIF1α stabilization, promoting anaerobic glycolysis, reducing ROS levels, and increasing the regenerative potential of more youthful HSCs. WT, wild-type. Professional illustration by Somersault18:24.
In hypoxic environments, loss of Sphk2 increases PDK3 expression via HIF1α stabilization, promoting anaerobic glycolysis, reducing ROS levels, and increasing the regenerative potential of more youthful HSCs. WT, wild-type. Professional illustration by Somersault18:24.
Still, Sphk2 has broad substrate specificity;6 thus, the potential implication of other Sphk2-phosphorylated targets requires additional investigation. Similarly, further research is warranted to determine how cell-intrinsic Sphk2 loss creates a chromatin-permissive environment for HIF1α to regulate the expression of different PDK enzymes. Finally, considering the known heterogeneity of HSCs,2 it is tempting to speculate that high Sphk2 might be a marker of functionally old HSCs. Additional single-cell studies should address this question.
Overall, Li et al unveiled a novel Sphk2-HIF1α-PDK3 axis as a checkpoint in the control of HSC metabolic fitness. This study represents a milestone in our understanding HSC metabolism/aging, adds Sphk2 to a growing list of master regulators of HSC functions,10 and sets the stage for future HSC rejuvenation strategies via boosting PDK3 expression, enhancing HIF1α stability, and/or targeting Sphk2.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal