Key Points
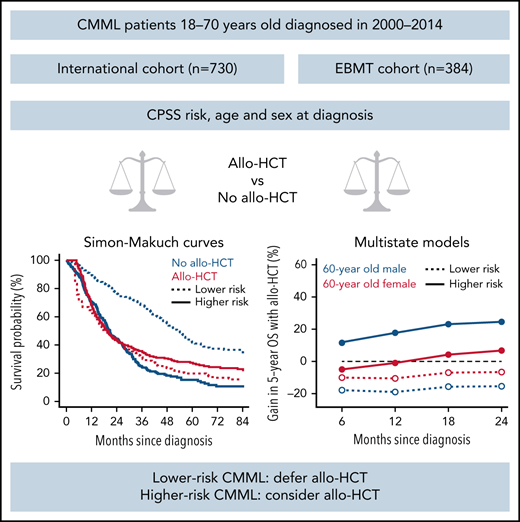
The survival benefit of allo-HCT was investigated in 1114 CMML patients with time-dependent analyses and multistate models.
Performing allo-HCT before transformation decreases life expectancy in lower-risk patients but may be considered in higher-risk patients.
Abstract
To determine the survival benefit of allogeneic hematopoietic cell transplantation (allo-HCT) in chronic myelomonocytic leukemias (CMML), we assembled a retrospective cohort of CMML patients 18-70 years old diagnosed between 2000 and 2014 from an international CMML dataset (n = 730) and the EBMT registry (n = 384). The prognostic impact of allo-HCT was analyzed through univariable and multivariable time-dependent models and with a multistate model, accounting for age, sex, CMML prognostic scoring system (low or intermediate-1 grouped as lower-risk, intermediate-2 or high as higher-risk) at diagnosis, and AML transformation. In univariable analysis, lower-risk CMMLs had a 5-year overall survival (OS) of 20% with allo-HCT vs 42% without allo-HCT (P < .001). In higher-risk patients, 5-year OS was 27% with allo-HCT vs 15% without allo-HCT (P = .13). With multistate models, performing allo-HCT before AML transformation reduced OS in patients with lower-risk CMML, and a survival benefit was predicted for men with higher-risk CMML. In a multivariable analysis of lower-risk patients, performing allo-HCT before transformation to AML significantly increased the risk of death within 2 years of transplantation (hazard ratio [HR], 3.19; P < .001), with no significant change in long-term survival beyond this time point (HR, 0.98; P = .92). In higher-risk patients, allo-HCT significantly increased the risk of death in the first 2 years after transplant (HR 1.46; P = .01) but not beyond (HR, 0.60; P = .09). Performing allo-HCT before AML transformation decreases life expectancy in lower-risk patients but may be considered in higher-risk patients.
Medscape Continuing Medical Education online
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1452.
Disclosures
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC, has disclosed the following relevant financial relationships: stock, stock options, or bonds: AbbVie Inc. (former).
Learning objectives
Upon completion of this activity, participants will:
• Describe the association of allogeneic hematopoietic cell transplantation (allo-HCT) and other factors with survival and other outcomes in chronic myelomonocytic leukemia (CMML), according to a retrospective cohort study
• Determine the effect of timing of allo-HCT on the association of allo-HCT and other factors with survival and other outcomes in CMML, according to a retrospective cohort study
• Identify clinical implications of the association of allo-HCT and other factors with survival and other outcomes in CMML, according to a retrospective cohort study
Release date: September 22, 2022; Expiration date: September 22, 2023
Introduction
Chronic myelomonocytic leukemia (CMML) is a rare disease predominantly affecting men, with a median age of 75 years at diagnosis. The prognosis remains unsatisfactory, with median overall survival (OS) of 30 to 36 months.1-5 Allogeneic hematopoietic cell transplantation (allo-HCT) is the only treatment recognized as potentially curative in CMML.6-12 However, allo-HCT in CMML patients is associated with a 20% to 50% 3-year nonrelapse mortality and a 25% to 60% cumulative incidence of relapse.6-12 The benefit of upfront allo-HCT over a nontransplant treatment thus remains questionable. Because CMML is a rare disease, transplantation indications and procedures are largely extrapolated from myelodysplastic syndromes (MDSs) or classical myeloproliferative neoplasms. In MDS, a first study reported that higher-risk patients had a survival benefit when transplanted upfront.13 More recent studies including prospective comparisons between allo-HCT and hypomethylating agents confirmed this finding.14-17 In CMML where the benefit of hypomethylating agents is less established,18,19 the question of transplantation is only relevant in the minority of patients young and fit enough for transplantation. Among patients younger than 65 years, Patnaik et al reported that 20% of them underwent transplantation, but the benefit of allo-HCT could not be analyzed in this series.20
The current study leverages the international CMML dataset (ICD)5 and the EBMT registry,9 applying statistical models taking into account timing of allo-HCT to study the role of allo-HCT in survival of CMML patients. This study, based on 2 large international cohorts, represents the first multicentric transplantation decision analysis in CMML.
Methods
Patients and study design
The ICD is a retrospective, multi-institution database assembled by the International Consortium for MDS/myeloproliferative neoplasms under the aegis of the MDS Foundation. Data on diagnosis, treatment, and outcome since CMML diagnosis after informed consent was collected from all participating centers after approval by each institution’s review board and centrally reviewed as previously published.5
Although ICD is informative with respect to the general prognosis and treatment of CMML, because these patients are rarely transplanted, we used additional data from the EBMT registry on survival after transplantation. EBMT is a nonprofit, scientific society representing >600 transplant centers located mainly in Europe. EBMT collects on a voluntary basis data on recipient and donor characteristics, treatment, and follow-up of patients undergoing blood and bone marrow transplantation.
To focus on allo-HCT eligible patients, the inclusion criteria for the present study for both ICD and EBMT registry patients were (1) CMML diagnosis according to World Health Organization (WHO) 2008 criteria21, (2) an age of 18 to 70 years at CMML diagnosis, (3) an Eastern Cooperative Oncology Group performance status <3 at CMML diagnosis, (4) CMML diagnosis between 2000 and 2014, (5) available follow-up data, including information on AML transformation (note that patients from the EBMT registry that died prior to the onset of allo-HCT conditioning were removed from the present analysis), and (6) available CMML Prognostic Scoring System (CPSS) risk category22 at CMML diagnosis (flowchart in supplemental Figure 1). The time of closure for the ICD and EBMT datasets were June 2014 and October 2019, respectively.
Definitions
CMML patients were stratified into myelodysplastic (CMML-MD) and myeloproliferative subsets based on the WHO cutoff of white blood cell > 13 × 109/L.21 Cytogenetic risk was classified as previously published.23 CPSS risk was calculated according to Such et al.22 Patients with low and intermediate-1 CPSS risk were grouped into the lower-risk category, and the remainder (CPSS intermediate-2 and high) in the higher-risk category. HLA matching was classified according to HLA allele high resolution on 10 antigens, and conditioning regimen intensity is defined as previously reported.24,25
Multistate models
We used multistate models to study the survival before and after allo-HCT and transformation to AML.26 The model includes the following states: diagnosis, AML, transplantation before transformation to AML, transplantation after transformation to AML, and death (distinguishing between death before and after AML transformation and allo-HCT but irrespective of precise cause of death). The models were used to predict probabilities for reference patients with specific combinations of baseline covariates. All patients start in the “Diagnosis” state. A patient remains in the current state until one of the modeled events (transformation to AML, allo-HCT, or death) occurs. Depending on the current state, a patient is at risk for subsequent events indicated by arrows from the current state (supplemental Figure 2). At the end of their follow-up, patients who have not reached an absorbing state (death) are censored for all transitions they are at risk for. At the time of an event, a patient transits from the current state to the new state depending on the event and remains there until a subsequent event or censoring occurs. We developed time inhomogeneous Markov multistate models, meaning that the hazard of transition from one state to another does not depend on the time spent in the current state but only on the current state and the time since diagnosis and that it is nonconstant. The effect of age and sex on the hazard of each transition is modeled using a semiparametric Cox proportional hazards model. Schoenfeld residuals are used to test for violations of the proportional hazards assumption. The relative differences are expressed in terms of hazard ratios (HRs).
Similar to predicting survival for a patient with certain risk factors from a Cox proportional hazards model, using multistate models, we can estimate the probability of a patient being in a certain state at a point in time, conditional on being in (possibly) another state at the time the prediction is made.26,27 The advantage of multistate models is that they can take competing risks and series of events into account.28 By default, predictions are made from time 0, when all patients are in the initial state (diagnosis), but the time of the prediction (the landmark time) can be varied just as the state from which the prediction is made. For example, when a patient comes for a follow-up visit, the prognosis can be updated using the information that the patient has developed transformation to AML, which is referred to as dynamic prediction.29 Alternatively, dynamic prediction can be used to study different scenarios on whether or when to transplant a patient by comparing the 5-year OS probability for a patient who, 1 year after diagnosis, is still in the diagnosis state vs a patient in the transplantation state. Note that although both example patients are conditioned on being alive 1 year after diagnosis, we would be comparing survival of patients not transplanted yet with the survival of patients who have undergone transplantation within the first year after diagnosis and have not died between allo-HCT and 1 year.
The model includes the following states: diagnosis, AML, transplantation before transformation to AML, transplantation after transformation to AML, and death. Based on the cumulative hazard plots, it was justified to assume that the transitions from diagnosis or AML to death, from diagnosis or AML to allo-HCT, and from allo-HCT (with and without prior transformation to AML) to death are all proportional and thus share the same baseline hazard functions. Age and sex effects were estimated in transition-specific Cox models. To avoid violation of the proportional hazards (PH) assumption, we fit the multistate model separately in the 2 CPSS risk groups.
Other statistical analyses
Unless otherwise specified, all survival times were measured starting at the date of CMML diagnosis, and ICD and EBMT registries were merged. To prevent selection bias, EBMT patients were included at the time of transplantation, with left truncation of pretransplant survival. Because the ICD included European centers that may have accrued transplanted patients to the EBMT registry, ICD transplanted patients were censored at the time of allo-HCT. Transplanted patients from ICD were used to estimate the probability to be transplanted but were not used to analyze posttransplant outcome. Hence, we assume that the pretransplant and posttransplant disease course can only be inferred from the ICD and the EBMT registry, respectively.
Median follow-up was calculated using the reverse Kaplan-Meier method.30 Survival probabilities were estimated using the Kaplan-Meier method. The log-rank test was used to compare the survival between CPSS risk groups. Cumulative incidences of AML transformation and allo-HCT were estimated in a competing-risks framework, also considering death (irrespective of cause) as competing event. The cumulative incidence of allo-HCT after AML transformation was calculated in a new competing-risks model with death as competing event, where the time was set to 0 at the time of AML transformation.
We used Simon-Makuch curves to visualize the (hypothetical) effect of undergoing transplantation immediately after diagnosis by CPSS risk group31 and tested with the Mantel-Byar method.32
We modeled the effect of transplantation on OS in a Cox proportional hazards model allowing different baseline hazards for the 2 CPSS risk groups. The model was adjusted for age and sex. Transformation to AML and allo-HCT were included as time-dependent covariates, and both main effects and interactions were estimated separately in the 2 risk groups. Schoenfeld residuals were used to investigate possible violations of the PH assumption. Because allo-HCT violated the PH assumption due to different prognostic impact in the longer vs shorter term, different HRs were estimated for the first 2 years after allo-HCT (to give an average short-/midterm effect) and the later period (2 years after allo-HCT and beyond). All analyses were performed in R version 3.6.2, using packages “survival,” “prodlim,” and “mstate.”27
Results
Patient characteristics
In total, 730 patients from ICD and 384 EBMT patients were included in the analysis (supplemental Figure 1). Median year of diagnosis was 2008 for ICD patients and 2011 for EBMT patients (Table 1). Patients from the ICD were older (median, 64 [ICD] vs 57 [EBMT] years). Most patients were male (69.6% and 69.3% in the ICD and EBMT registry, respectively). The proportion of patients with CMML-MD (ICD, 47.7%; EBMT, 45.1%) and with low cytogenetic risk (ICD, 68.2%; EBMT, 68.8%) was similar in the 2 cohorts. CPSS risk at diagnosis was lower and higher in 386 (52.9%) and in 344 (47.1%) ICD patients, respectively; 177 (46.1%) and 207 (53.4%) EBMT patients had lower and higher CPSS risk at diagnosis, respectively. At the time of allo-HCT, 50 of 312 (16%) evaluable EBMT patients had bone marrow blasts ≥10%, whereas 121 of 170 evaluable patients (71.2%) with <10% bone marrow blasts had either red blood cell or platelet transfusion dependence. Of the 78 EBMT patients having transformed to AML before allo-HCT, 40 (51.3%) had achieved complete remission before allo-HCT. Reassessment of CPSS at the time of allo-HCT was only available in 172 (44.8%) EBMT patients (detailed in supplemental Table 2) and thus could not be accounted for. In the EBMT cohort, 140 patients (36.8%) were transplanted with an HLA-matched sibling donor, 21 (5.5%) with an HLA-mismatched related donor, 144 (37.9%) with an HLA-matched unrelated donor, 59 (15.5%) with an HLA-mismatched unrelated donor, 16 (4.2%) were transplanted with an unrelated donor without HLA information, and information was missing for 4 (0.1%) patients (supplemental Table 1).
Patient characteristics and main events
| . | ICD cohort (n = 730) . | EBMT cohort (n = 384) . |
|---|---|---|
| Year of diagnosis | 2008 (2004-2010) | 2011 (2008-2013) |
| Age at diagnosis, years | 64.0 (58.8-67.8) | 57.3 (51.0-61.9) |
| Sex | ||
| Male | 508 (69.6) | 266 (69.3) |
| Female | 222 (30.4) | 118 (30.7) |
| FAB subsets | ||
| CMML-MD | 348 (47.7) | 173 (45) |
| CMML-MP | 382 (52.3) | 211 (55) |
| WHO category* | ||
| CMML-1 | 582 (79.7) | 238 (62) |
| CMML-2 | 148 (20.3) | 146 (38) |
| Hemoglobin level | ||
| ≥10 gr/dl | 472 (64.7) | 235 (61.2) |
| <10 gr/dl | 258 (35.3) | 149 (38.8) |
| Cytogenetic risk† | ||
| Low | 498 (68.2) | 264 (68.7) |
| Intermediate | 113 (15.5) | 46 (12.0) |
| High | 119 (16.3) | 74 (19.3) |
| CPSS risk‡ | ||
| Low | 159 (21.8) | 61 (15.9) |
| Intermediate-1 | 227 (31.1) | 116 (30.2) |
| Intermediate-2 | 286 (39.2) | 162 (42.2) |
| High | 58 (7.9) | 45 (11.7) |
| Follow-up duration from diagnosis, months (median, 95% CI) | 51.06 (47.34-56.77) | 78.03 (67.61-84.07) |
| Underwent allo-HCT | 98 (13.4) | 384 (100) |
| Transformation to AML before allo-HCT | 33 (33.7)§ | 78 (20.3) |
| . | ICD cohort (n = 730) . | EBMT cohort (n = 384) . |
|---|---|---|
| Year of diagnosis | 2008 (2004-2010) | 2011 (2008-2013) |
| Age at diagnosis, years | 64.0 (58.8-67.8) | 57.3 (51.0-61.9) |
| Sex | ||
| Male | 508 (69.6) | 266 (69.3) |
| Female | 222 (30.4) | 118 (30.7) |
| FAB subsets | ||
| CMML-MD | 348 (47.7) | 173 (45) |
| CMML-MP | 382 (52.3) | 211 (55) |
| WHO category* | ||
| CMML-1 | 582 (79.7) | 238 (62) |
| CMML-2 | 148 (20.3) | 146 (38) |
| Hemoglobin level | ||
| ≥10 gr/dl | 472 (64.7) | 235 (61.2) |
| <10 gr/dl | 258 (35.3) | 149 (38.8) |
| Cytogenetic risk† | ||
| Low | 498 (68.2) | 264 (68.7) |
| Intermediate | 113 (15.5) | 46 (12.0) |
| High | 119 (16.3) | 74 (19.3) |
| CPSS risk‡ | ||
| Low | 159 (21.8) | 61 (15.9) |
| Intermediate-1 | 227 (31.1) | 116 (30.2) |
| Intermediate-2 | 286 (39.2) | 162 (42.2) |
| High | 58 (7.9) | 45 (11.7) |
| Follow-up duration from diagnosis, months (median, 95% CI) | 51.06 (47.34-56.77) | 78.03 (67.61-84.07) |
| Underwent allo-HCT | 98 (13.4) | 384 (100) |
| Transformation to AML before allo-HCT | 33 (33.7)§ | 78 (20.3) |
Main events
Median follow-up from diagnosis was 51 months in ICD and 78 months in EBMT cohort (Table 1). Ninety-eight (13.4%) ICD patients were transplanted, of whom 33 were transformed into AML before allo-HCT. Transformation to AML before allo-HCT occurred in 78 (20.3%) EBMT patients. In the ICD cohort (censored at allo-HCT), the 5-year cumulative incidences of AML were 22% (95% confidence interval [CI], 17% to 27%) and 36% (95% CI, 30% to 41%) in lower and higher CMML-risk patients, respectively (supplemental Figure 3).
In the ICD, 5-year OS from diagnosis was 40% (95% CI, 34-47) in lower-risk patients and 20% (95% CI, 15% to 25%) in higher risk (supplemental Figure 4). Five-year OS from transplantation was similar in EBMT patients with lower-risk CMML (5-year OS, 33%; 95% CI, 25-41) and those with higher risk at diagnosis (5-year OS, 33%; 95% CI, 24-40; P = .76, supplemental Figure 5). Of note, there was no significant difference in posttransplant OS between patients transplanted from an HLA-matched donor and those transplanted with other donor types (P = .22, supplemental Figure 6) and between those transplanted after a standard- or a reduced-intensity conditioning regiment (P = .6, supplemental Figure 7).
Univariable analysis of the impact of allo-HCT
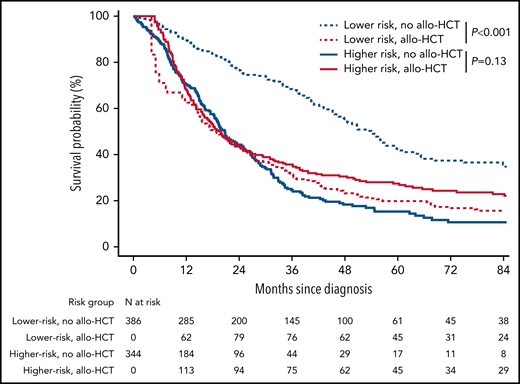
To analyze the impact of allo-HCT according to CPSS risk, we first considered allo-HCT as a time-dependent parameter in the global cohort, where pretransplant data are left-truncated in EBMT patients and posttransplant censored in ICD patients. Figure 1 presents Simon-Makuch curves for OS after allo-HCT according to risk category determined at CMML diagnosis. Lower-risk patients had a 5-year OS of 20% (95% CI, 12% to 33%) with allo-HCT vs 42% (95% CI, 35% to 49%) without allo-HCT (Mantel-Byar P < .001). In higher-risk patients, 5-year OS was 27% (95% CI, 21% to 34%) with allo-HCT vs 15% (95% CI, 11% to 22%) without allo-HCT (Mantel-Byar P = .13, Figure 1).
Simon-Makuch survival curves according to CMML risk group. Simon-Makuch survival curves representing the effect of transplantation for the hypothetical scenario where all patients are transplanted immediately after diagnosis vs never transplanted according to the CMML risk group. Lower risk: CPSS low or intermediate-1; higher risk: CPSS intermediate-2 or high. P values from Mantel-Byar tests.
Simon-Makuch survival curves according to CMML risk group. Simon-Makuch survival curves representing the effect of transplantation for the hypothetical scenario where all patients are transplanted immediately after diagnosis vs never transplanted according to the CMML risk group. Lower risk: CPSS low or intermediate-1; higher risk: CPSS intermediate-2 or high. P values from Mantel-Byar tests.
Of note, patients alive 24 months after a diagnosis of lower-risk CMML and transplanted within this time frame were significantly younger than those alive 24 months after diagnosis without allo-HCT (transplanted n = 79, median age 57.7 years vs nontransplanted n = 200, median age 64 years; P < .001), but these 2 patients populations were otherwise comparable at CMML diagnosis in terms of gender, WHO category, French-American-British subset, hemoglobin level, and cytogenetic risk (all P >.05, supplemental Table 3).
Multistate models
Multistate models were next used to estimate the potential advantage of allo-HCT when performed at different times from diagnosis. Supplemental Figure 2 shows the multistate model structure along with data used to estimate each transition. The ICD cohort was used to inform the course of the disease until allo-HCT, including probability to be transplanted or death before allo-HCT. EBMT data were used to estimate the transitions from transplant to death. Transition-specific covariate effect estimates are available in Table 2, where hazard ratios indicate the relative risk of transitioning to a given state (AML transformation, allo-HCT, or death) according to current state (diagnosis, AML transformation, or allo-HCT), age (as a continuous variable), and sex. The same multistate model was estimated separately in lower- and higher-risk CMML patients because CMML risk violated the proportional hazards assumption. These estimates can then be used to predict the probability for a given reference patient to be in each state at different time points, as exemplified for a 60-years-old male (Figure 2) or female (supplemental Figure 8) patient by CPSS risk group.
Transition-specific covariate effect estimates in the multistate model according to CMML risk at diagnosis
| Covariates . | Transition . | Lower-risk CMML . | Higher-risk CMML . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| AML vs diagnosis state | To death | 4.99 (3.23-7.72) | <.001 | 3.60 (2.51-5.16) | <.001 |
| Allo-HCT from AML vs from diagnosis | To death | 1.49 (0.86-2.59) | .159 | 1.19 (0.68-2.07) | .547 |
| AML vs diagnosis state | To allo- HCT | 8.34 (3.97-17.50) | <.001 | 5.48 (2.60-11.52) | <.001 |
| Female vs male | Diagnosis to AML | 0.78 (0.46-1.33) | .367 | 0.72 (0.47-1.11) | .135 |
| Age at diagnosis* | Diagnosis to AML | 1.07 (0.77-1.49) | .683 | 0.79 (0.66-0.94) | .008 |
| Female vs male | Diagnosis to allo-HCT | 1.11 (0.55-2.24) | .766 | 1.40 (0.68-2.89) | .359 |
| Age at diagnosis* | Diagnosis to allo-HCT | 0.47 (0.35-0.64) | <.001 | 0.60 (0.45-0.78) | <.001 |
| Female vs male | Diagnosis to death | 0.69 (0.45-1.05) | .082 | 0.73 (0.51-1.06) | .099 |
| Age at diagnosis* | Diagnosis to death | 1.14 (0.87-1.48) | .347 | 1.29 (1.01-1.64) | .045 |
| Female vs male | AML to allo-HCT | 0.44 (0.10-1.97) | .280 | 0.90 (0.32-2.53) | .844 |
| Age at diagnosis* | AML to allo-HCT | 0.64 (0.40-1.02) | .058 | 0.72 (0.51-1.01) | .056 |
| Female vs male | AML to death | 0.74 (0.37-1.46) | .383 | 0.87 (0.52-1.44) | .583 |
| Age at diagnosis* | AML to death | 1.15 (0.77-1.70) | .494 | 1.16 (0.88-1.52) | .289 |
| Female vs male | Allo-HCT (prior to AML) to death | 0.55 (0.33-0.93) | .025 | 1.24 (0.85-1.87) | .317 |
| Age at diagnosis* | Allo-HCT (prior to AML) to death | 1.17 (0.87-1.58) | .304 | 1.05 (0.87-1.26) | .625 |
| Female vs male | Allo-HCT (post-AML) to death | 0.54 (0.17-1.64) | .274 | 0.83 (0.31-2.22) | .708 |
| Age at diagnosis* | Allo-HCT (post-AML) to death | 1.83 (0.74-4.52) | .192 | 1.09 (0.67-1.79) | .729 |
| Covariates . | Transition . | Lower-risk CMML . | Higher-risk CMML . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| AML vs diagnosis state | To death | 4.99 (3.23-7.72) | <.001 | 3.60 (2.51-5.16) | <.001 |
| Allo-HCT from AML vs from diagnosis | To death | 1.49 (0.86-2.59) | .159 | 1.19 (0.68-2.07) | .547 |
| AML vs diagnosis state | To allo- HCT | 8.34 (3.97-17.50) | <.001 | 5.48 (2.60-11.52) | <.001 |
| Female vs male | Diagnosis to AML | 0.78 (0.46-1.33) | .367 | 0.72 (0.47-1.11) | .135 |
| Age at diagnosis* | Diagnosis to AML | 1.07 (0.77-1.49) | .683 | 0.79 (0.66-0.94) | .008 |
| Female vs male | Diagnosis to allo-HCT | 1.11 (0.55-2.24) | .766 | 1.40 (0.68-2.89) | .359 |
| Age at diagnosis* | Diagnosis to allo-HCT | 0.47 (0.35-0.64) | <.001 | 0.60 (0.45-0.78) | <.001 |
| Female vs male | Diagnosis to death | 0.69 (0.45-1.05) | .082 | 0.73 (0.51-1.06) | .099 |
| Age at diagnosis* | Diagnosis to death | 1.14 (0.87-1.48) | .347 | 1.29 (1.01-1.64) | .045 |
| Female vs male | AML to allo-HCT | 0.44 (0.10-1.97) | .280 | 0.90 (0.32-2.53) | .844 |
| Age at diagnosis* | AML to allo-HCT | 0.64 (0.40-1.02) | .058 | 0.72 (0.51-1.01) | .056 |
| Female vs male | AML to death | 0.74 (0.37-1.46) | .383 | 0.87 (0.52-1.44) | .583 |
| Age at diagnosis* | AML to death | 1.15 (0.77-1.70) | .494 | 1.16 (0.88-1.52) | .289 |
| Female vs male | Allo-HCT (prior to AML) to death | 0.55 (0.33-0.93) | .025 | 1.24 (0.85-1.87) | .317 |
| Age at diagnosis* | Allo-HCT (prior to AML) to death | 1.17 (0.87-1.58) | .304 | 1.05 (0.87-1.26) | .625 |
| Female vs male | Allo-HCT (post-AML) to death | 0.54 (0.17-1.64) | .274 | 0.83 (0.31-2.22) | .708 |
| Age at diagnosis* | Allo-HCT (post-AML) to death | 1.83 (0.74-4.52) | .192 | 1.09 (0.67-1.79) | .729 |
Because some pairs of transitions were assumed to be proportional, they share the same baseline hazard function. The relative difference of the hazards, within a pair, is expressed in terms of hazard ratio. For example, the hazard of death (without/before transplantation) after transformation to AML for lower-risk patients is estimated to be 5 times as high as the hazard of death without transformation to AML. Similarly, an effect of transformation to AML was estimated on the hazard of transplantation and the hazard of death after transplantation. Additionally, transition-specific age and sex effects were modeled. The same multistate model was estimated separately in lower- and higher-risk CMML patients as CPSS risk violated the proportional hazards assumption.
Age by unit of 10 years.
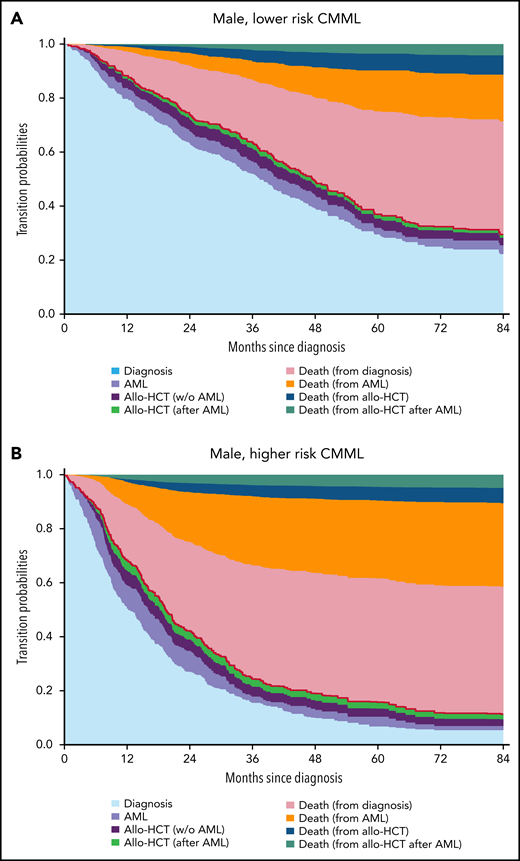
Transition probabilities in a 60-year-old male patient. Transition probabilities predicted from the multistate model for 60-year-old male patients in (A) the lower-risk CMML group and (B) the higher-risk CMML group. At each point in time, the distance between 2 adjacent curves represents the probability of being in the corresponding state. The probability of being in an intermediate state can both increase and decrease over time, whereas the probability of absorbing (death) states can only increase over time. Additionally, the order of the states is such that the figure shows predicted OS curves: the sum of the probabilities of being in the states diagnosis, transformation to AML (AML), and treatment with allogeneic stem cell transplantation before AML (w/o AML) and after AML (after AML) transformation to AML, indicated by the red line, equals the probability of being alive (ie, OS). Similar transition probabilities for 60-years-old females are provided in supplemental Figure 8. w/o, without.
Transition probabilities in a 60-year-old male patient. Transition probabilities predicted from the multistate model for 60-year-old male patients in (A) the lower-risk CMML group and (B) the higher-risk CMML group. At each point in time, the distance between 2 adjacent curves represents the probability of being in the corresponding state. The probability of being in an intermediate state can both increase and decrease over time, whereas the probability of absorbing (death) states can only increase over time. Additionally, the order of the states is such that the figure shows predicted OS curves: the sum of the probabilities of being in the states diagnosis, transformation to AML (AML), and treatment with allogeneic stem cell transplantation before AML (w/o AML) and after AML (after AML) transformation to AML, indicated by the red line, equals the probability of being alive (ie, OS). Similar transition probabilities for 60-years-old females are provided in supplemental Figure 8. w/o, without.
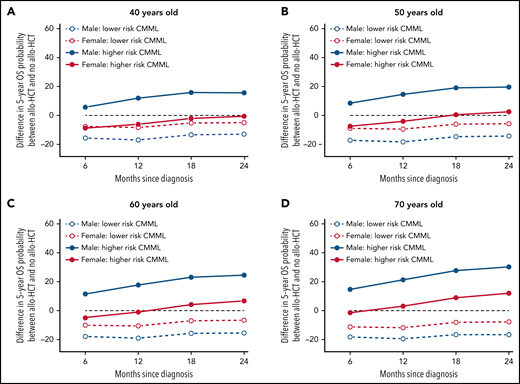
Expectedly, most patients moved from diagnosis to another state (death, AML, allo-HCT) during follow-up. The transition probabilities from diagnosis to AML and to death were lower in patients with lower-risk CMML at diagnosis than those with higher risk, older patients had a higher risk of AML, AML transformation increased the risk of dying, and most patients died without undergoing allo-HCT. Figure 3 shows the model-based benefit or loss of OS provided by performing allo-HCT before transformation to AML for different reference patients characterized by age, sex, and CPSS risk, conditional on performing transplantation (and surviving) within the first 6, 12, 18, or 24 months from diagnosis. Performing allo-HCT was always detrimental in patients with lower-risk disease at diagnosis, regardless of age, sex, and interval between diagnosis and transplantation. The survival benefit of allo-HCT in CMML patients with higher-risk disease at diagnosis was mostly confined to men, without clear survival gain or loss in women, except women 60 years or older transplanted and alive 18 months after diagnosis or later. A similar modeling analysis was applied to estimate the change in OS in patients receiving allo-HCT after AML transformation (supplemental Figure 9). Performing allo-HCT in this context always provided a survival advantage, regardless of age, sex, and disease risk at CMML diagnosis.
Change in 5-year OS probabilities with allo-HCT prior to AML transformation, compared with no allo-HCT, derived from the multistate models with landmarks by 6 months from diagnosis to 24 months. The multistate model was used to predict 5-year OS for reference patients defined by patient sex, age at diagnosis, and CPSS risk group at diagnosis in (A) 40-year-old, (B) 50-year-old, (C) 60-year-old, and (D) 70-year-old patients, respectively, for landmark times 6, 12, 18, and 24 months since diagnosis (x-axis). Predictions are conditional on surviving without transformation to AML until the landmark time. At each landmark time, the difference between the 5-year OS predicted for patients who have undergone allo-HCT without prior transformation to AML at any time between diagnosis and the landmark time and that of patients who have not transformed to AML and have not been transplanted (yet) is displayed in the y-axis. Positive values indicate advantage of undergoing allo-HCT, and negative values suggest that not-transplanted patients have better prognosis.
Change in 5-year OS probabilities with allo-HCT prior to AML transformation, compared with no allo-HCT, derived from the multistate models with landmarks by 6 months from diagnosis to 24 months. The multistate model was used to predict 5-year OS for reference patients defined by patient sex, age at diagnosis, and CPSS risk group at diagnosis in (A) 40-year-old, (B) 50-year-old, (C) 60-year-old, and (D) 70-year-old patients, respectively, for landmark times 6, 12, 18, and 24 months since diagnosis (x-axis). Predictions are conditional on surviving without transformation to AML until the landmark time. At each landmark time, the difference between the 5-year OS predicted for patients who have undergone allo-HCT without prior transformation to AML at any time between diagnosis and the landmark time and that of patients who have not transformed to AML and have not been transplanted (yet) is displayed in the y-axis. Positive values indicate advantage of undergoing allo-HCT, and negative values suggest that not-transplanted patients have better prognosis.
Multivariable Cox models for survival
The potential advantage of allo-HCT was further studied in Cox models considering allo-HCT and transformation to AML as time-dependent covariates and adjusting for CPSS risk at diagnosis, age (as a continuous variable), and sex. Consistent with the multistate model, older age and male sex were significantly associated with a higher risk of mortality (Table 3). In lower-risk patients, performing allo-HCT before transformation to AML significantly increased the risk of death in the first 2 years after allo-HCT (HR, 3.19; 95% CI, 2.30-4.42; P < .001), without providing a significant long-term benefit beyond this time point (HR, 0.98; 95% CI, 0.58-1.64; P = .92). In higher-risk patients, allo-HCT performed before transformation was detrimental within 2 years from allo-HCT (HR, 1.46; 95% CI, 1.09-1.96; P = .01), with a nonsignificant trend toward survival benefit beyond 2 years (HR, 0.60; 95% CI, 0.34-1.08; P = .09). Comparatively, the overall benefit of allo-HCT was much clearer in patients transplanted after transforming to AML, regardless of their CPSS risk category at CMML diagnosis (Table 3). Focusing on patients transplanted with matched related or unrelated donors led to similar results (supplemental Table 4). When inspecting each disease-related parameter included in the CPSS (white blood cell count, anemia, bone marrow blast count, and cytogenetic risk), none of these CPSS components identified by itself a subset of patients with unequivocal survival benefit of allo-HCT (ie, lack of significant OS loss in the first 2 years after allo-HCT) and significant OS benefit after 2 years (supplemental Table 5).
Multivariable analysis of OS
| . | Hazard ratio . | (95% CI) . | P . |
|---|---|---|---|
| Patient sex: female vs male | .80 | (0.67-0.96) | .017 |
| Age at diagnosis* | .12 | (1.02-1.24) | .023 |
| Lower-risk patients | |||
| Td AML | .14 | (3.63-7.28) | <.001 |
| Td allo-HCT (without AML): 0-2 y | .19 | (2.30-4.42) | <.001 |
| Td allo-HCT (without AML): >2 y | .98 | (0.58-1.64) | .924 |
| Td allo-HCT (after AML): 0-2 y | .89 | (0.53-1.50) | .675 |
| Td allo-HCT (after AML): 2+ y | .20 | (0.0.07-0.56) | .002 |
| Higher-risk patients | |||
| Td AML | .68 | (2.74-4.92) | <.001 |
| Td allo-HCT (without AML): 0-2 y | .46 | (1.09-1.96) | .012 |
| Td allo-HCT (without AML): 2+ y | .60 | (0.34-1.08) | .089 |
| Td allo-HCT (after AML): 0-2 y | .45 | (0.28-0.73) | .001 |
| Td allo-HCT (after AML): 2+ y | .08 | (0.0.02-0.33) | .001 |
| . | Hazard ratio . | (95% CI) . | P . |
|---|---|---|---|
| Patient sex: female vs male | .80 | (0.67-0.96) | .017 |
| Age at diagnosis* | .12 | (1.02-1.24) | .023 |
| Lower-risk patients | |||
| Td AML | .14 | (3.63-7.28) | <.001 |
| Td allo-HCT (without AML): 0-2 y | .19 | (2.30-4.42) | <.001 |
| Td allo-HCT (without AML): >2 y | .98 | (0.58-1.64) | .924 |
| Td allo-HCT (after AML): 0-2 y | .89 | (0.53-1.50) | .675 |
| Td allo-HCT (after AML): 2+ y | .20 | (0.0.07-0.56) | .002 |
| Higher-risk patients | |||
| Td AML | .68 | (2.74-4.92) | <.001 |
| Td allo-HCT (without AML): 0-2 y | .46 | (1.09-1.96) | .012 |
| Td allo-HCT (without AML): 2+ y | .60 | (0.34-1.08) | .089 |
| Td allo-HCT (after AML): 0-2 y | .45 | (0.28-0.73) | .001 |
| Td allo-HCT (after AML): 2+ y | .08 | (0.0.02-0.33) | .001 |
Effect estimates from multivariable Cox proportional hazards model allowing different baseline hazards for lower and higher CPSS risk groups. The model was adjusted for age and sex. Transformation to AML and transplantation were included as time-dependent (Td) covariates. To overcome violations of the proportional hazards assumption, the effect of allo-HCT was split into an average short-/midterm effect covering the first 2 years after transplantation and an average long-term effect covering the period beyond 2 years after allo-HCT. The latter is estimated in patients who are alive at 2 years since allo-HCT
Unit 10 years
Discussion
In this large-scale study combining 2 CMML registry cohorts, we report that transplanting patients diagnosed with lower-risk disease is detrimental. Univariable and multivariable time-dependent analyses found a nonsignificant trend for improved OS in higher-risk patients. According to our multistate model, men with higher-risk CMML benefit from allo-HCT regardless of its timing, whereas a survival benefit with allo-HCT is restricted to women 60 years or older when transplant is delayed. Finally, allo-HCT is always beneficial in patients having transformed to AML.
A recent German multicenter study retrospectively comparing the outcome of 119 transplanted patients to that of 142 nontransplanted patients, adjusting for age, found a clear survival benefit for allo-HCT in higher-risk patients but not in lower-risk patients.33 Another single-center study of 70 transplanted patients matched 1-to-1 with nontransplanted patients to adjust for age and CMML risk suggested an OS benefit with allo-HCT.34
Compared with prior reports, our study accounted for the time-dependent impact of allo-HCT on survival and the selection process from diagnosis to allo-HCT. We studied the impact of allo-HCT by using univariable Simon-Makuch and multivariable Cox models considering allo-HCT and transformation to AML as time-dependent covariates. This approach allows to account for the exact timing of allo-HCT. However, Cox models only yield relative (HR) rather than absolute (survival probabilities) risk estimations. This limitation was overcome by a multistate model, a method ideally suited to study the impact of postdiagnosis events and interventions and thus already applied to analyze outcome after allo-HCT,28,35 including in MDS.13,14,36-38 Our multistate model further expands on those reports by allowing flexible, time-dependent hazards. Overall, these methods limit the biases inherent to retrospective comparisons of transplanted vs nontransplanted patients.33,34
Results from our models must nevertheless be interpreted with caution. For instance, the large survival benefit associated with allo-HCT in patients having transformed to AML could reflect the selection of patients achieving remission prior to allo-HCT and the known high mortality of AML secondary to CMML39 and thus the fact that the only survivors are those who received an allogeneic transplantation, even though few patients could receive an allo-HCT in this situation. As we and others have reported, the long-term survival of patients who transformed from myeloproliferative disease remains poor and should encourage transplantation before transformation.40,41
While EBMT patients are prospectively accrued from the date of transplantation, the ICD is a retrospective registry from diagnosis.5,9 Consequently, the ICD may underestimate mortality rate (alive patients may have been more frequently reported) and EBMT misses patients planned for transplantation but never receiving it. ICD and EBMT recruited patients from different centers, even if some were overlapping. We adjusted our analysis on the main prognostic factors to balance the potential differences between both registries. Because a proportion of ICD patients received allo-HCT, we could estimate the probability to be transplanted, in contrast to studies comparing transplant and nontransplant cohort, where the transplant eligibility of patients in the nontransplant cohort is often unknown.
Our study used CPSS to stratify patients at diagnosis because it relies on broadly available parameters, including cytogenetics, and has been externally validated.22 Though several other clinical prognostic models have been applied to CMML,4,42-46 none has shown superiority over CPSS.5 Future studies including comorbidity indexes, splenomegaly, and other extramedullary disease, as well as somatic mutations, which were available only in a small minority of transplanted patients in the present study, may delineate CMML subsets benefiting from allo-HCT.2,3,47-49 Conversely, neither donor type nor conditioning regimen intensity impacted posttransplant survival overall, and these variables were thus not accounted for in our models. Further studies will be required to explore the potential survival with matched donors in lower-risk patients or of haplo-identical donors in higher-risk cases. The latter could not be captured over the time period (2000-2014) of the present study.
The expected under-representation of females in our cohort makes estimation of allo-HCT benefit less precise in this population. Moreover, the overall better outcome of females, regardless of allo-HCT, may account for the less-robust benefit of allo-HCT noted in females compared with males in our study. Differences in comorbidities, pathogenesis, and response to nontransplant therapies may also have contributed to this differential effect.50,51
Repeated assessment of disease risk during follow-up, prior to transplantation or transformation to AML, would certainly have refined both time-dependent Cox models and multistate modeling. The limited data available in the EBMT cohort suggest that a third of lower-risk patients were referred to transplantation because of progression to higher risk. However, aside from younger age (accounted for in multistate and multivariable models), baseline disease characteristics seemed comparable in transplanted vs nontransplanted lower-risk disease. Future studies accounting for progression to higher-risk disease before allo-HCT and not only transformation to AML may refine this analysis, though contrary to several MDS prognostic systems,52,53 the dynamic use (ie, after diagnosis) of CPSS or other CMML prognostic models has yet to be validated. Conversely, patients with higher-risk disease may have received treatments before undergoing transplantation. Although nontransplant therapies have yet to show robust disease-modifying potential in CMML, future studies will need to account for the dynamic landscape of these options.18,19
Our analyses highlighted the increased risk of death within the first 2 years posttransplant. Such biphasic posttransplant mortality has notably been reported in MDS and attributed to transplant-related mortality caused by early complications, including graft rejection, acute graft-versus-host disease, infection, and organ failures in registries and in prospective studies.14,17,54 Ongoing progresses in the management of these complications may decrease this early mortality and nuance our conclusions.15,16,55,56 Future studies should also consider extensive chronic graft-versus-host disease in multivariable Cox and multistate models to account for quality of life after allo-HCT.57
Overall, our study supports current recommendations, largely based on expert opinion and previous MDS studies,14,36,37 to defer allo-HCT in lower-risk patients and consider it carefully in those with higher-risk disease.58 Any other practice modification should be undertaken in the context of a clinical trial.
Acknowledgments
The authors thank the clinical consortium of the MDS Evans Foundation and the International Working Group for Myeloproliferative Neoplasms for serving as the platform for the development of the International CMML Dataset. The authors acknowledge the contribution of all the EBMT and ICD centers contributing patients to this study.
Authorship
Contribution: M.R., L.C.d.W., and R.I. designed the study and drafted the manuscript; L.d.W. supervised the statistical analyses performed by K.B. and D.-J.E.; L.K. managed data from the EBMT registry; E.P., P.F., A.N., R.K.R., R.S.K., S.B.K., E.S., G.G.-M., M.P.M., and F.O. accrued patients to the ICD; D.W.B., J.S., N.G., A.R., J.F., V.P., F.L., A.B., V.R., P.H., and F.O. accrued patients to the EBMT registry; I.Y.-A. chairs the chronic malignancies working party of EBMT; and all authors reviewed the manuscript and approved its final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie Robin, Service Hématologie Greffe de Moelle, Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, F-75010 Paris, France; e-mail: marie.robin@aphp.fr; and Raphael Itzykson, Service Hématologie Adultes, Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, F-75010 Paris, France; e-mail: raphael.itzykson@aphp.fr.
This study has been presented in part at the 2019 Annual Meeting of the American Society of Hematology and the 2020 Annual Meeting of EBMT.
Send data sharing requests via e-mail to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal