Key Points
Patients with a slow early response by PET1 have a significantly higher rate of relapse, which can be mitigated by adding 21-Gy IFRT.
With relapses common in the PET1+ site, there is a role for up-front alternative systemic therapy and/or target RT intensification.
Abstract
Children’s Oncology Group (COG) trial AHOD0431 reduced systemic therapy and used response-adapted involved-field radiotherapy (IFRT) in early-stage pediatric classic Hodgkin lymphoma. We investigated the impact of positron emission tomographic response after 1 cycle (PET1) and on IFRT outcomes and pattern of relapse. Patients in AHOD0431 underwent PET1 response assessment after AVPC (doxorubicin, vincristine, prednisone, and cyclophosphamide). “Rapid early responders” (RERs) had a negative PET1 (PET1−); “slow early responders” (SERs) had a positive PET1 (PET1+). Patients with a partial response by computed tomographic and functional imaging after 3 chemotherapy cycles received 21-Gy IFRT, whereas complete responders had no IFRT. Progression-free survival (PFS) was evaluated for RERs and SERs treated with or without IFRT. Recurrence sites were initial, new, or both. Relapses involving initial sites were characterized as “within the PET1+ site” or “initially involved but outside the PET1+ site.” Median follow-up was 118 months. The 10-year PFS rate among RERs was 96.6% with IFRT and 84.1% without IFRT (P = .10), whereas SERs were 80.9% with IFRT and 64.0% without IFRT (P = .03). Among 90 RERs who did not receive IFRT, all 14 relapses included an initial site. Among 45 SERs receiving no IFRT, 14 of 16 relapses were in the initial site (9 PET1+ site only). Among 58 patients receiving IFRT, 5 of 10 relapses were in the PET1+ site. After 3 cycles of AVPC alone, RERs showed favorable results. Conversely, SERs had unfavorable outcomes with AVPC alone, although they improved with 21-Gy IFRT. RT remains an important component of treatment for SERs. This trial was registered at www.clinicaltrials.gov as #NCT00302003.
Introduction
Pediatric early-stage classic Hodgkin lymphoma (cHL) is highly curable in most children1; however, the risk of late toxicity from chemotherapy and radiation therapy is significant.2,3 In an effort to mitigate the late effects, treatment strategies have been developed to maintain high cure rates while minimizing the risk of treatment-related toxicities. These strategies have included using less-intensive chemotherapy regimens and response-adapted protocols to identify patients for whom radiotherapy (RT) can be omitted.4-12
To de-escalate treatment, the Children’s Oncology Group developed AHOD0431 for early-stage, low-risk cHL. The study examined whether reducing up-front treatment, by using a response-based approach with minimal initial chemotherapy and omission of involved-field RT (IFRT), in patients with a complete response (CR) could maintain overall survival and event-free survival (EFS) while reducing late toxicity. Unfortunately, the study did not meet its prespecified EFS benchmark in the context of AVPC (doxorubicin, vincristine, prednisone, and cyclophosphamide) chemotherapy and response-adapted RT. The investigators found that a slow early response (SER) at the first positron emission tomography scan after 1 cycle (PET1) was prognostic of outcomes and associated with significantly worse EFS in the absence of IFRT, despite having a CR at the end of chemotherapy. In an effort to inform future RT dose and field design, the purpose of this report is to explore the impact of a positive PET1 (PET1+) response and the use of IFRT on outcomes and patterns of relapse.
Methods
Eligibility
Details of the AHOD0431 protocol, including RT and chemotherapy regimens, have been reported.9 In brief, from February 2006 through April 2009, 278 eligible patients from age 0 to 21 years with low-risk cHL, defined as Ann Arbor stage IA and IIA nonbulky disease, provided informed consent and enrolled in an institutional review board–approved study protocol. Bulk was defined as a mediastinal mass greater than one-third of the longest thoracic dimension on an upright posterior-anterior chest x-ray or any contiguous nodal aggregate measuring >6 cm across the longest transverse diameter on axial imaging.
Treatment
The initial treatment regimen consisted of three 21-day cycles of AVPC chemotherapy including doxorubicin 25 mg/m2 on days 1 and 2, vincristine 1.4 mg/m2 (maximum, 2.8 mg) on days 1 and 8, prednisone 20 mg/m2 twice daily on days 1 through 7, and cyclophosphamide 600 mg/m2 on days 1 and 2, given every 21 days with growth factor support.
Evaluation
In the original study schema, patients underwent response assessment by functional imaging after 1 cycle of AVPC and by functional and computed tomographic (CT) imaging after 3 cycles. Treatment, however, was adapted based only on the end-of-treatment (EOT) functional and CT imaging after 3 cycles. As initially defined for the protocol, patients with a CR after chemotherapy did not receive consolidative IFRT, whereas those with a partial response (PR) received 21 Gy of IFRT in 1.5-Gy fractions over 14 days, delivered 3 to 4 weeks after the final cycle of chemotherapy. IFRT volumes included sites initially involved by disease delivered with balanced anterior-posterior fields.
Functional imaging assessment included fluorodeoxyglucose-PET (PET1) or gallium scan, and a CR was defined as activity below or at the level of the mediastinal blood pool. CT imaging criteria for a CR was a ≥80% reduction in the product of the perpendicular dimension and no residual extramediastinal lymph node mass >2.0 cm. A PR was defined as ≥50% but <80% decrease in perpendicular dimension on CT or positive fluorodeoxyglucose-PET or gallium scan. All response determinations were reviewed centrally at the Imaging and Radiation Oncology Core Quality Assurance Review Center (Lincoln, RI). Patients with a negative PET1 were not required to have PET3 imaging performed. Details regarding the salvage regimen are provided in a published study.9
AHOD0431 was temporarily closed to accrual on 4 December 2008 because of an increased risk of relapse among PET1+ patients who did not receive IFRT because they had achieved a CR, with a recommendation that all patients with equivocal or positive PET1 receive 21-Gy IFRT, unless they were >12 months from completion of chemotherapy. The study was permanently closed on 3 April 2009, when the time from study entry to the time of IFRT dropped below the prespecified goal of 65%.
Present analysis
For relapses, the following images were reviewed in a central location: baseline imaging at diagnosis to determine initial site(s) of disease; interim and postchemotherapy functional (PET1 and PET3) and anatomic imaging (CT); a radiation treatment plan, if applicable; and imaging at relapse. Sites of recurrence were categorized as initial, new, or both, and for the PET1+ subgroup as PET1+ site or initially involved site beyond the PET1+ site (Figure 1). The Kaplan-Meier method was used to evaluate progression-free survival (PFS) by PET1, and the log-rank test was used for comparisons between survival curves.
Pattern of relapse definitions. (A) Theoretical outline (pink) of initial site of disease based on the prechemotherapy PET/CT scan. A relapse within the outlined region would have been categorized as a relapse in the “initial site” of disease. A relapse outside of the outlined region would be considered a “new site” of relapse. (B) Theoretical outline (blue) of fluorodeoxyglucose-avid residual disease greater than the mediastinal blood pool on PET/CT after 1 cycle of AVPC. For PET1+ patients, a relapse within the blue region would be considered a relapse at the “PET1+ site,” whereas a relapse outside of the blue region but within the pink region from panel A would be considered “initial site beyond the PET1+ site.”
Pattern of relapse definitions. (A) Theoretical outline (pink) of initial site of disease based on the prechemotherapy PET/CT scan. A relapse within the outlined region would have been categorized as a relapse in the “initial site” of disease. A relapse outside of the outlined region would be considered a “new site” of relapse. (B) Theoretical outline (blue) of fluorodeoxyglucose-avid residual disease greater than the mediastinal blood pool on PET/CT after 1 cycle of AVPC. For PET1+ patients, a relapse within the blue region would be considered a relapse at the “PET1+ site,” whereas a relapse outside of the blue region but within the pink region from panel A would be considered “initial site beyond the PET1+ site.”
Results
The original study report included 278 patients with 52 relapses.9 The current analysis is restricted to 222 patients who underwent PET1 and, if positive, a subsequent PET3. Therefore, 56 patients were excluded: 45 nonrelapsing patients without a PET1 available and 11 relapsing patients (7 with gallium imaging or unavailable relapse scans, 2 without PET1 completed, 1 without PET3 completed, and 1 with progression during chemotherapy). Among the 222 participants, 41 (18%) relapses occurred. Up-front IFRT was considered RT delivered before any documented relapse and included patients called back for IFRT related to an SER after the temporary study closure. Patients receiving salvage IFRT for relapse were considered not to have received up-front IFRT.
The median follow-up was 118 months for the 222 patients, and only 2 patients died during follow-up, both of whom had relapsed. The median time to relapse was 10.02 (range, 4.11-32.39) months for all relapses. For RERs it was 10.83 months if they did not receive RT and 6.57 months if they received RT, whereas for SERs it was 8.46 months if they did not receive RT and 21.40 months if they received it. Patient characteristics are shown in Table 1. According to PET1 response, 54% (n = 119) of patients were RERs and 46% (n = 103) were SERs. Only 3 patients had a PR by PET/CT scan after 3 cycles of AVPC chemotherapy. SERs were more likely at baseline to be female (P = .032) and to have stage II disease (P = .0017), an erythrocyte sedimentation rate >20 (P = .0001), and 3 or more sites of disease (P = .0052; supplemental Table 1, available on the Blood Web site).
Characteristics of eligible patients with or without relapsed disease upon diagnosis
| Characteristic . | Relapse (n = 41) . | No relapse (n = 181) . |
|---|---|---|
| Sex | ||
| Male | 23 (56.10) | 76 (41.99) |
| Female | 18 (43.90) | 105 (58.01) |
| Race | ||
| White | 31 (75.60) | 145 (80.11) |
| Black | 4 (9.76) | 19 (10.50) |
| Other | 3 (7.32) | 2 (1.10) |
| Unknown | 3 (7.32) | 15 (8.29) |
| Median age (y) | 15.56 [6.69, 21.76] | 15.25 [3.08, 21.94] |
| Stage | ||
| I | 7 (17.07) | 47 (25.97) |
| II | 34 (82.93) | 134 (74.03) |
| Pathology by central review | ||
| NS | 30 (73.17) | 115 (63.54) |
| MC | 2 (4.88) | 30 (16.57) |
| Missing | 9 (21.95) | 36 (19.89) |
| ESR | ||
| ≤20 | 14 (34.15) | 109 (60.22) |
| >20 | 27 (65.85) | 72 (39.78) |
| CRP | ||
| ≤2× upper limit | 23 (56.10) | 135 (74.59) |
| >2× upper limit | 18 (43.90) | 46 (25.41) |
| Disease sites, n | ||
| 1-2 | 35 (85.37) | 164 (90.61) |
| ≥3 | 6 (14.63) | 17 (9.39) |
| Characteristic . | Relapse (n = 41) . | No relapse (n = 181) . |
|---|---|---|
| Sex | ||
| Male | 23 (56.10) | 76 (41.99) |
| Female | 18 (43.90) | 105 (58.01) |
| Race | ||
| White | 31 (75.60) | 145 (80.11) |
| Black | 4 (9.76) | 19 (10.50) |
| Other | 3 (7.32) | 2 (1.10) |
| Unknown | 3 (7.32) | 15 (8.29) |
| Median age (y) | 15.56 [6.69, 21.76] | 15.25 [3.08, 21.94] |
| Stage | ||
| I | 7 (17.07) | 47 (25.97) |
| II | 34 (82.93) | 134 (74.03) |
| Pathology by central review | ||
| NS | 30 (73.17) | 115 (63.54) |
| MC | 2 (4.88) | 30 (16.57) |
| Missing | 9 (21.95) | 36 (19.89) |
| ESR | ||
| ≤20 | 14 (34.15) | 109 (60.22) |
| >20 | 27 (65.85) | 72 (39.78) |
| CRP | ||
| ≤2× upper limit | 23 (56.10) | 135 (74.59) |
| >2× upper limit | 18 (43.90) | 46 (25.41) |
| Disease sites, n | ||
| 1-2 | 35 (85.37) | 164 (90.61) |
| ≥3 | 6 (14.63) | 17 (9.39) |
Data are the number of patients (percentage of the study group), unless otherwise indicated.
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MC, mixed cellularity; NS, nodular sclerosing.
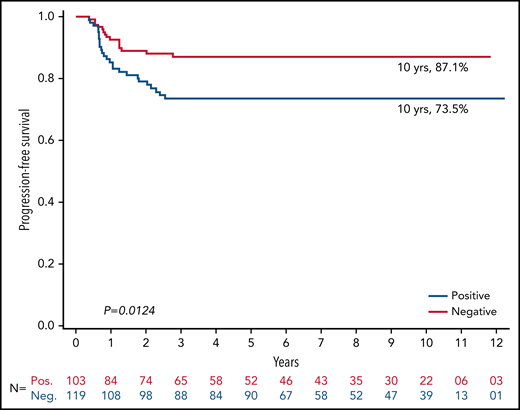
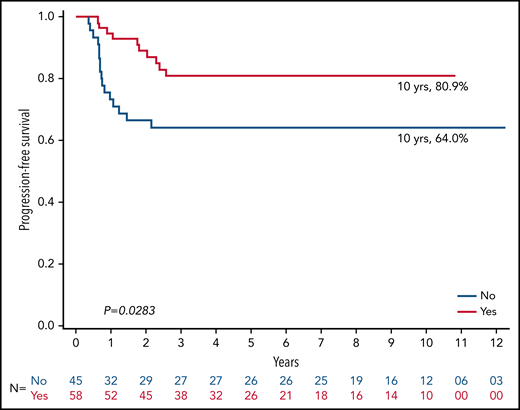
The PFS rate at 10 years was 87.1% for RERs and 73.5% for SERs (P = .01; Figure 2). The 10-year PFS rate among 119 RERs was 96.6% among those who received IFRT (n = 29) vs 84.1% among those who did not receive IFRT (n = 90; P = .10; Figure 3). The 10-year PFS rate among 103 SERs was 80.9% for those who received IFRT (n = 58) vs 64.0% for those who did not receive IFRT (n = 45; P = .03; Figure 4).
PFS among PET1− patients stratified by receipt of up-front IFRT. n = 119.
PFS among PET1− patients stratified by up-front IFRT or not. n = 103.
Patterns of relapse according to PET1 response and IFRT
Table 2 reports the patterns of relapse according to PET1 response and use of IFRT. Among the recurring RERs (n = 15), an initial site of disease was involved in all recurrences. Among 14 patients who did not receive IFRT, sites of relapse included only an initial site in 11 (78%) patients and an initial plus a new site of disease in 3 (21%). The RER who underwent IFRT after a PR to AVPC had a recurrence in the initial irradiated site of disease only.
Sites of relapse among patients who were PET1− or PET1+: no up-front RT vs up-front RT
| Site of relapse . | PET 1−, n = 119* . | PET 1+, n = 103† . | ||
|---|---|---|---|---|
| No IFRT, n (%) n = 14 . | IFRT, n (%) n = 1 . | No IFRT, n (%) n = 16 . | IFRT, n (%) n = 10 . | |
| Pet 1 (+) site‡ | — | — | 9 (56.3) | 5 (50) |
| Initial site | 11 (78) | 1 (100) | — | — |
| PET 1 (+) and initial site‡ | — | — | 5 (31.3) | 0 (0) |
| Initial site beyond PET1+ site‡ | — | — | 0 (0) | 1 (10) |
| Initial and new site | 3 (21) | 0 (0) | 2 (12.5) | 2 (20) |
| New site only | 0 (0) | 0 (0) | 0 (0) | 2 (20) |
| Site of relapse . | PET 1−, n = 119* . | PET 1+, n = 103† . | ||
|---|---|---|---|---|
| No IFRT, n (%) n = 14 . | IFRT, n (%) n = 1 . | No IFRT, n (%) n = 16 . | IFRT, n (%) n = 10 . | |
| Pet 1 (+) site‡ | — | — | 9 (56.3) | 5 (50) |
| Initial site | 11 (78) | 1 (100) | — | — |
| PET 1 (+) and initial site‡ | — | — | 5 (31.3) | 0 (0) |
| Initial site beyond PET1+ site‡ | — | — | 0 (0) | 1 (10) |
| Initial and new site | 3 (21) | 0 (0) | 2 (12.5) | 2 (20) |
| New site only | 0 (0) | 0 (0) | 0 (0) | 2 (20) |
PET1− patients were considered RERs.
PET1+ patients were considered SERs.
Pertinent to PET1+ patients only.
Among all 26 SERs who had recurrence (16 no IFRT, 10 IFRT), 24 (92%) had relapse at an initial site and 54% (n = 14) solely at the PET1+ site. Among the 16 patients who did not undergo IFRT, 14 (88%) had recurrence within the initial site of disease, 9 of whom had it solely in the PET1+ site, 5 with recurrence in the PET1+ site and outside the PET1+ site but all within the initial site, and 2 (12%) with recurrence in an initial and a new site of disease. Specific to the 10 patients who had a relapse after up-front IFRT, 6 patients (60%) had relapse within an initial site of disease that was irradiated, 5 of whom experienced a recurrence solely within the PET1+ site. The other sites of relapse included initial and new sites of disease in 2 patients (20%) and new sites of disease alone in 2 patients (20%). Three of the 10 SERs also had a positive PET3, and all 3 of them had recurrence within the PET3+ site.
Discussion
Unfortunately, the AHOD0431 study did not achieve its intent of overall de-escalation of treatment in early-stage cHL because of the high number of patients needing RT and poor outcomes for the SERs. However, the present study demonstrates the importance of RT among patients with early-stage cHL who have an SER based on interim PET1 after 3 cycles of AVPC chemotherapy. Furthermore, the sites of relapse among SERs suggest the need to further optimize therapy for patients with positive PET1 with alternative chemotherapy or immunotherapy and/or increasing the RT dose to PET1+ sites. Finally, within the context of the present chemotherapy regimen, a negative EOT PET3, even when CT criteria for CR were met, was not sensitive enough to detect microscopic disease remaining in initial sites of disease and could not be used to decrease treatment intensity without clear loss of disease control.
HL survivors are at a great risk for late complications from both chemotherapy and RT. Because of the excellent cure rate with combined-modality therapy, recent studies have focused on ways to minimize treatment-related morbidity, either through less-intensive chemotherapy or by reducing the radiation dose. Over the past 15 years, the most popular paradigm has been PET response–adapted treatment, which has often focused on eliminating the use of RT for RERs and intensifying treatment for SERs (supplemental Table 2).4-17 Although, in the present study, early PET response was used to adapt therapy initially, the results demonstrated that, in this treatment paradigm, the PET1 response was a more sensitive predictor of PFS than the end-of-chemotherapy CT and PET response. This finding resulted in SERs being called back for IFRT if they had completed chemotherapy within the prior year, even if they met the criteria for a CR by PET and CT imaging at completion of systemic therapy.
When evaluating outcomes in AHOD0431 for patients who were RER by PET1, it is evident that those who received combined-modality therapy (10-year PFS, 96.6%) and chemotherapy alone (10-year RFS, 84.1%) compare favorably with patients enrolled in the 3 other major trials, conducted to analyze the role of PET2 or PET3 in guiding treatment reduction for early-stage cHL in adults and using chemotherapy of comparable intensity (supplemental Table 2). In the EORTC H10 study, which used interim PET2 to determine treatment adaptation, favorable-risk patients (H10F) who received 3 cycles of ABVD followed by 30-Gy IFRT in the standard arm had a 5-year PFS rate of 99.0% compared with a 5-year PFS rate of 87.1% among those in the experimental arm with a negative PET2 who received 4 cycles of ABVD without RT (P < .05).18 In the United Kingdom RAPID study, patients with a negative EOT PET3 randomized to ABVD ×3 followed by 30-Gy IFRT had a 3-year PFS rate of 97.1% compared with a 3-year PFS rate of 90.8% with ABVD ×3 alone, which did not meet the trial’s prespecified noninferiority margin.19 More recently, the German Hodgkin Study Group HD16 trial used an EOT PET2-guided approach to de-escalate RT in the experimental arm.17 The 5-year PFS rate was 93.4% among patients with negative PET2 in the standard arm who received 2 cycles of ABVD followed by 20-Gy IFRT compared with a 5-year PFS rate of 86.1% among those who received ABVD ×2 alone in the experimental PET-adapted arm. Again, the authors reported a clinically meaningful reduction in PFS with the omission of RT. Importantly, in-field recurrences within the hypothetical RT field drove the increase in PFS among those patients who did not receive IFRT (9% vs 2% for ABVD alone vs CMT; P < .01), a finding similar to that in the present AHOD0431 cohort as well as in the H10 and RAPID trials.
Although outcomes among the RER cohort enrolled in AHOD0431 resemble those in other studies concerning adults who achieved an RER, of note, a much lower percentage of patients (54%) would have been eligible for omission of RT in AHOD0431 based on PET1 compared with the trials that used PET2 or PET3 and RER rates of 66% to 91% (the highest rates of RER among studies using Deauville 4 as the cutoff for SER rather than Deauville 3). Consequently, although the PET1 response can be prognostic, the potential proportion of patients able to benefit from treatment deescalation may be reduced compared with when PET2 or PET3 is used or if a higher Deauville score is used as the cutoff for SERs. The distinction becomes crucial when trying to de-escalate therapy in a larger proportion of patients. Conversely, however, the results from AHOD0431 also demonstrate that treatment adaptation based on EOT CT and PET3 can result in unacceptably low PFS in the context of AVPC.
AHOD0431 was unique in being the only study that allowed omission of RT for SERs (PET1+). With a 10-year PFS rate of 80.9% with IFRT and 64% without IFRT among SERs (P < .05), our results clearly demonstrated both the importance of IFRT for disease control in SERs with interim PET1+ and the prognostic significance of PET1. As such, no comparable cohort exists for our SER cohort who did not receive IFRT. Most relapses in the SER cohort of AHOD0431 occurred within the initial site of disease and within the slowly responding site that was PET1+. This finding not only reinforces the importance of consolidative RT among patients with less-favorable disease but also suggests a potential benefit from dose intensification above 21 Gy to the PET1+ region to further improve outcomes. Among SERs, a higher radiation dose delivered as a selective tailored boost to the small-volume PET1+ sites may reduce in-field recurrences without increasing radiation-related toxicities. Just as important, alternative chemotherapy or immunotherapy may also be beneficial in SERs to reduce out-of-field recurrences, which occurred in approximately one-quarter of them, regardless of IFRT.20 Similar approaches are being taken by the Euronet-PHL-C1 study (registered on https://clinicaltrials.gov as #NCT 00433459), which treats low-, intermediate-, and high-risk group SER sites to 30.6 Gy,21 Euronet-PHL-C2 study (#NCT 02684708), which treats intermediate- and high-risk SERs up to 29.8 Gy, and the Pediatric Classical Hodgkin Lymphoma Consortium study cHOD17 (#NCT03755804), which treats intermediate- and high-risk SERs with 25.5 Gy.22 Although no randomized trials evaluating doses of 20 Gy vs 30 Gy in this setting for cHL exist, there may be an opportunity for the Euronet group to compare local control between the PHL-C1 low-risk SER cohort with a Deauville 4 or 5 response who received a boost to the PET+ site vs the PHL-C2 low-risk SER cohort, which only received 19.8 Gy. Another potential option for management of SER patients is to combine RT with more intensive chemotherapy, such as the adaptive treatment approach used in the EORTC H10 study with escBEACOPP with RT.18 Alternatively, one could consider a chemoimmunotherapy with RT, such as in KEYNOTE-667 (#NCT03407144), a collaboration with Children’s Oncology Group, is a study wherein children and young adults with favorable early-stage HL receive 2 cycles of ABVD chemotherapy. Those with a PR (Deauville 4/5) move on to pembrolizumab + AVD ×2 followed by consolidative involved-site RT with a boost to sites of PET+ disease at completion of chemotherapy. Whether to manage SERs with more aggressive systemic therapy or higher doses of RT must be individualized to each patient through a discussion including the radiation oncologist, hematologist, and the patient (or patient’s parents) based on the anticipated risks of side effects from each treatment approach according to the disease site requiring treatment.
The AVPC regimen used in this study was developed from the regimen used in the GPOH- HD95 study,5 wherein we substituted cyclophosphamide at 1200 mg/m2 per course for the etoposide, to introduce an alkylating agent and increase the intensity of therapy. This approach was used to achieve higher CR rates and eliminate etoposide without causing gonadal toxicity or increasing the risk of alkylating agent-related secondary leukemia. The relative contribution of the specific chemotherapy regimen used in this study compared with other regimens is complicated by differences in patient eligibility and study design. ABVD has been widely used in adult HL trials, but the pediatric experience is relatively limited. In the current trial, the number of disease sites and erythrocyte sedimentation rate were not included in eligibility considerations, leading to a higher proportion of patients with unfavorable risk factors than the favorable group enrolled in the EORTC H10 trial. Similarly, the ABVE-PC regimen reported by Friedman et al (AHOD0031) examined a population of patients with intermediate-risk features that were mutually exclusive to the concurrently run AHOD0431 trial.10 Small series using the ABVD regimen in the pediatric population have been published with good outcomes and limited use of radiation.23 The authors acknowledge that optimization of the chemotherapy regimen may lead to improved overall outcomes, although the observations related to RT use would be likely to remain unchanged. Currently, the National Comprehensive Cancer Network guidelines24 for low-risk pediatric HL continue to support the use of OEPA ×2 among RERs, whereas SERs proceed to IFRT to all sites with a boost to sites of inadequate response per the GPOH-200225 and EuroNet-PHL-C1 study.26
The present study is not without its limitations. The entire analysis was exploratory and did not reflect specific up-front planned end points. As such, the subgroup analyses based according to PET1 and RT were not initially planned and should be carefully interpreted considering the small sample size in some subgroups. Finally, positioning these results within the context of other response-adapted trials is a challenge given the various inclusion criteria, RT doses, and chemotherapy regimens used (supplemental Table 2). Specifically, patients enrolled on United Kingdom RAPID trials most likely comprise a higher risk group than those on the EORTC H10F and GHSG HD16 trials and therefore may be most comparable to patients on the present AHOD0431 study. Both in the United Kingdom RAPID and AHOD0431 studies, no restriction was placed on the number of involved nodal sites for patients with stage II disease, and nearly one-third of the patients in both these trials would not have met criteria for inclusion in GHSG HD16.
PET response after 1 cycle of AVPC chemotherapy was a significant predictor for treatment outcome after 3 cycles of AVPC in children with early-stage HL. Among patients who were SER based on PET1, IFRT improved PFS significantly and should remain a component of treatment for this patient population with less-favorable outcomes in the present treatment paradigm. Based on the pattern of relapse, SERs may benefit from additional intensification of systemic therapy and a targeted RT boost to the PET1+ site to a dose greater than 21 Gy. Both of these features will be integrated into the next cooperative group trial for low- and intermediate-risk pediatric HL.
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Cancer Institute (NCI) grants to the Children’s Oncology Group: Chair’s Grant U10 CA98543, National Clinical Trials Network (NCTN) Operations Center Grant U10 CA180886, and the NCTN Statistics and Data Center Grant U10 CA180899, Imaging and Radiation Core (IROC) Grant U24 CA180903; the Princess Margaret Cancer Foundation; and St Baldrick’s Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: A.P. performed the research and collected, analyzed, and interpreted the data and drafted the manuscript; F.G.K. designed the research and analyzed and interpreted the data; K.M.M.C. performed the research, analyzed and interpreted the data, contributed analytical tools, and wrote the manuscript; S.K. and S.C. collected data and contributed analytical tools; Q.P. analyzed and interpreted the data, performed the statistical analysis, and wrote the manuscript; Y.W. analyzed and interpreted the data and performed the statistical analysis; S.M.C. designed the research, contributed analytical tools, collected the data, and wrote the manuscript; L.S.C. and C.L.S. designed the research and analyzed and interpreted the data; D.H. and K.M.K. designed the research, analyzed and interpreted the data, and wrote the manuscript; B.S.H. designed and performed the research and collected, analyzed, and interpreted the data and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.P. is Department of Radiation Oncology, Boston University School of Medicine, Boston, MA.
Correspondence: Bradford S. Hoppe, Department of Radiation Oncology, Mayo Clinic, 4500 San Pablo Rd S, Jacksonville, FL 32224; e-mail: hoppe.bradford@mayo.edu.
The authors agree to share anonymized data with researchers per Children's Oncology Group guidelines.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal