In this issue of Blood, Chong et al1 report safety and efficacy data on the administration of the programmed cell death protein 1 (PD-1) inhibitor pembrolizumab to patients with refractory or relapsed large B-cell lymphoma (LBCL) after CD19-directed chimeric antigen receptor T-cell (CAR19) therapy.
CAR19 therapies induce impressive responses in chemorefractory patients and have upended the treatment paradigm for LBCL. Three CAR19 products are now approved by the U.S. Food and Drug Administration. Similarly, encouraging response rates have led to recent approvals in mantle cell and follicular lymphoma. The first non-CAR19 product, targeting the B-cell maturation antigen, has been approved for multiple myeloma. However, our justified excitement over these therapies must be tempered by the reality that durable remissions are seen in only 30% to 40%2 of patients with LBCL. Therefore, strategies to enhance CAR19 efficacy and address posttreatment disease progression represent a critical unmet need.
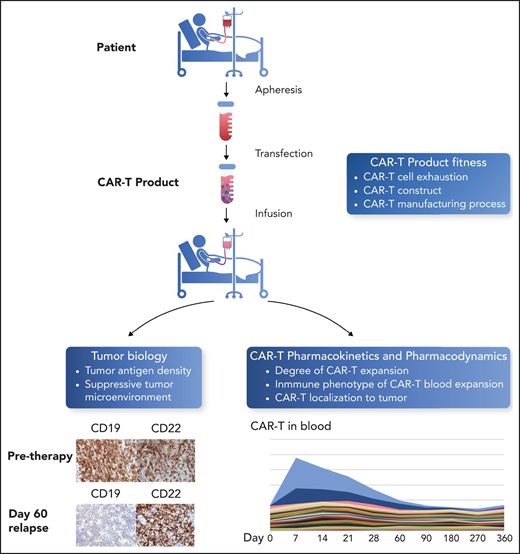
One of the major causes of CAR T-cell therapy failure is T cell exhaustion, a state in which prolonged exposure to antigen culminates in T cell dysfunction (see figure).3 T-cell exhaustion is associated with surface expression of inhibitory molecules, such as PD-1 and cytotoxic T-lymphocyte–associated protein 4. Antibodies that block checkpoint molecules have revolutionized the treatment of solid tumors and are now approved in a wide array of cancer types. Preventing or reversing CAR T-cell exhaustion is of great importance in the field of cellular therapy, and checkpoint inhibitors represent an ideal class of drugs for clinical testing.
Parameters for success of CAR T-cell therapy. The success of CAR T-cell therapy depends on the interplay of multiple factors, including T cell fitness before apheresis and after the manufacturing process; the ability of CAR T cells to expand after infusion, both in the blood and at the site of disease; and the characteristics of the tumor, including density of the target antigen on tumor cells and the immunosuppressive nature of the tumor microenvironment. Figure adapted from a slide provided by David Miklos (Stanford, University, Stanford, CA). Professional illustration by Somersault18:24.
Parameters for success of CAR T-cell therapy. The success of CAR T-cell therapy depends on the interplay of multiple factors, including T cell fitness before apheresis and after the manufacturing process; the ability of CAR T cells to expand after infusion, both in the blood and at the site of disease; and the characteristics of the tumor, including density of the target antigen on tumor cells and the immunosuppressive nature of the tumor microenvironment. Figure adapted from a slide provided by David Miklos (Stanford, University, Stanford, CA). Professional illustration by Somersault18:24.
In the phase 1 trial conducted by Chong and colleagues, pembrolizumab, a humanized monoclonal antibody targeting PD-1, was given to 12 patients with treatment failure after CAR19. Interestingly, 75% were primary refractory to CAR19 therapy, which typically induces responses in 70% to 80% of patients. Overall, pembrolizumab was well tolerated and met its safety end point. The main grade ≥3 toxicity was neutropenia and was seen in 25% of patients, which is important, as many CAR19-treated patients have prolonged cytopenias. Three patients (25%) had either a complete or partial response, with 1 further patient deriving clinical benefit, achieving stable disease.
Chong et al also performed translational studies to examine biomarker correlates of responses. CAR19 cells were detectable in the blood before initiation of the trial and reexpanded in 10 of 12 patients after pembrolizumab. Although peak CAR-T cell levels were not associated with response, responding patients were observed to have multiple episodes of CAR-T cell reexpansion. To examine this phenomenon further, Chong et al performed highly detailed T-cell phenotyping by using mass cytometry. They found that CAR T cells differed from non-CAR+ T cells, because of higher levels of exhaustion markers. Patients responding to pembrolizumab, when compared with nonresponding patients, had CAR T cells with lower expression of exhaustion-associated markers. Similarly, non-CAR T cells in responding patients also appeared to be less exhausted. These data suggest that tumor responses could be mediated through persistent CAR19 or through bystander non-CAR+ T cells. In addition, higher levels of exhaustion of T cells in nonresponding patients suggest there may be a threshold beyond which checkpoint inhibition is insufficient to reinvigorate T-cell function.
Although this study provides hope for a patient population with few available treatment options, important questions remain. T-cell exhaustion appears to be a significant contributor to CAR T-cell resistance in LBCL, but immune evasion by decrease or loss of CD19 after CAR19 therapy has been reported in ∼30%4 of patients with relapsed or progressive disease. CAR19 resistance related to target antigen loss is not likely to be improved by checkpoint therapy and will necessitate alternative strategies, such as multiantigen-targeting CAR constructs.5 In addition to antigen loss, other changes in the tumor microenvironment may adversely impact the efficacy of checkpoint inhibitors. High levels of systemic inflammatory markers, such as ferritin and interleukin-6, have been associated with decreased CAR19 responses.6 Recently, these markers were associated with a tumor interferon gene signature that has been reported as a negative prognostic factor in solid tumors treated with checkpoint inhibitors.7 The current study found that responders had lymphoma with 10% to 50% expression of PD-L1; because of the small sample, Chong et al did not observe a difference between responding and nonresponding patients.
Chong et al found evidence of exhaustion of CAR T cells at the time of progression. However, the problem of CAR T-cell exhaustion may arise earlier in the course of disease. Patients with hematologic malignancies have more terminally differentiated T cells than do healthy controls.8 Studies of CAR T-cell products have shown that more differentiated T cells at baseline may result in poor proliferative capacity during manufacturing; conversely, apheresis material enriched for naive or memory phenotypes have shorter product-doubling times and are associated with more durable responses after normalizing to tumor burden.6
The CAR T-cell manufacturing process itself also can contribute to T-cell exhaustion, as differences in growth media, choice of T-cell activator, and duration of activation can affect the acquisition of both phenotypic and metabolic hallmarks of T-cell dysfunction. Strategies to rest CAR-T cells during the manufacturing process, such as protocols wherein the cells are cultured in the presence of the tyrosine kinase inhibitor dasatinib,9 have been incorporated to promote a more favorable CAR T-cell phenotype.10 The preemptive use of checkpoint inhibitors in combination with CAR19 is also the subject of multiple studies to prevent CAR T-cell exhaustion in vivo.
The Russian chess grandmaster Savielly Tartakower is thought to have said, “Moral victories do not count.” For the hematologist whose patient with refractory LBCL achieves a remission, but subsequently relapses, the result is also a hollow “victory.” In chess, the term gambit is used to describe an opening that results in the loss of a piece but yields a strategic advantage. CAR19 therapies, in their current state, have been a brilliant opening move as we advance adoptive cellular immunotherapies for hematologic cancers. The challenge that faces us is to convert the high proportion of remissions seen with CAR T-cell therapies into ultimate victories. Better strategies to follow our successful opening must address challenges that include CAR T-cell exhaustion and target downregulation and microenvironment hostility and will ultimately get us from check to checkmate.
Conflict-of-interest disclosure: K.V.K. has been an ad hoc advisor to Kite/Gilead, Novartis, Celgene, JNJ, Autolus, Kiadis, Incyte, Gamida Cell, Roche, Cimeio, Avacta Therapeutics, Takeda, United Health Care, and Iovance and a noncompensated member of the Board of Directors of the National Marrow Donor Program/Be The Match. J.Y.S. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal