TO THE EDITOR:
Multiple myeloma (MM) is considered a high-priority condition for vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 The immune response to the vaccine is uncertain.2 Because high titers of neutralizing antibodies (nAbs) are necessary to inhibit the delta variant of the virus,3 patients with impaired immune response may be at risk of breakthrough infection. We prospectively investigated the humoral and cellular immunologic response to anti–SARS-CoV-2 messenger RNA (mRNA) vaccine (Pfizer-BioNTech BNT162b2) in patients with MM who were receiving therapy or in whom treatment was discontinued within 12 months (from January 2021) at a single tertiary care institution in Paris.
Among 72 consecutive patients with MM, 11 had a history of previous SARS-CoV-2 infection. At the time of vaccination, 48 patients were being treated with an anti-CD38 immunotherapy-based regimen (anti-CD38 group), and 24 patients were not being treated (non–anti-CD38 group) (Figure 1A). These 2 groups were well-balanced for variables that included age, sex, International Staging System score, high-risk cytogenetics, disease duration, a history of autologous hematopoietic stem cell transplantation, and concurrent therapy with immunomodulatory imide drugs and/or proteasome inhibitors. However, patients receiving anti-CD38 therapy had more treatment lines, were more frequently remote from autologous hematopoietic stem cell transplantation, and had lower gammaglobulin levels compared with non–anti-CD38 patients (supplemental Table 1). Serum samples were collected the day before vaccination (T0), before the second dose (M1), and 1 to 2 months after completion of vaccination (M3) (see supplemental Methods). An informed signed consent was obtained from each patient in agreement with the Declaration of Helsinki. We measured the production of anti-spike (S) immunoglobulin G (IgG) and IgA and nAbs against alpha (B.1.1.7) and delta (B.1.617.2) variants using S-Flow and S-Fuse neutralization assays, respectively.4,5 Moreover, we quantified the proportion of SARS-CoV-2–specific interferon-γ (IFN-γ)–producing T cells ex vivo among patient samples by using EliSpot after stimulation with S1 and S2 antigens at M3. Results obtained from samples from patients with MM were compared with those obtained from 23 healthy volunteers (control group; median age, 53 years; range, 25-73 years).
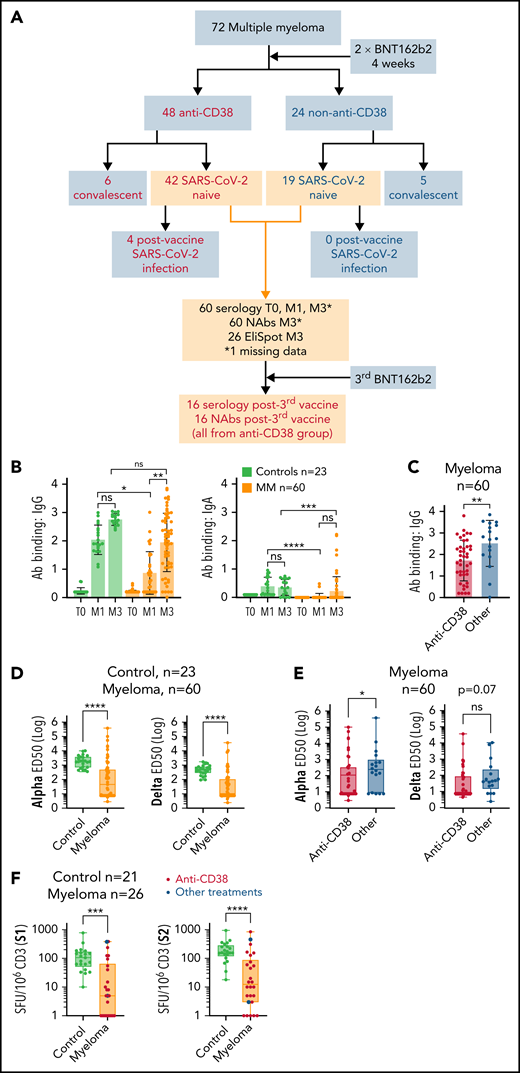
Impaired humoral and cellular response to BNT162b2 in patients with MM. (A) Flowchart of the study. BNT162b2 anti–SARS-CoV-2 vaccine was used in this study; 2 doses were administrated 4 weeks apart. In all, 16 patients (from the anti-CD38 group) received a third booster vaccine dose. The anti-CD38 group is defined as patients who were treated with anti-CD38 immunotherapy. The convalescent group is defined as patients with a history of SARS-CoV-2 infection who were given the vaccine. T0 is the time before vaccine, M1 is 1 month after the first vaccine dose, and M3 is 3 months after the first vaccine dose. (B-C) SARS-CoV-2–specific IgG and IgA production quantified by S-Flow in 60 patients with MM who had never been infected with SARS-CoV-2 and 23 healthy volunteers. (B) IgG (left panel) and IgA (right panel) quantification in controls or patients with MM before vaccination (T0) and at M1 and M3. (C) Comparison of IgG amounts in patients with MM who were treated or not treated with anti-CD38 immunotherapy. (D-E) Quantification of anti-SARS-CoV-2 nAbs against alpha or delta variants in controls (n = 23) or patients with MM (n = 60). (D) Comparison between controls and patients with MM. (E) Comparison between patients receiving or not receiving anti-CD38 immunotherapy. (F) Quantification of cellular immune response by S1 or S2 EliSpot (in spot forming units, SFU, per 106 CD3 cells) in controls (n = 21) or patients with MM (n = 26). Patients receiving anti-CD38 immunotherapies are indicated. Error bars represent standard error. *P < .05; **P < .01; ***P < .001; ****P < .0001. ns, not significant.
Impaired humoral and cellular response to BNT162b2 in patients with MM. (A) Flowchart of the study. BNT162b2 anti–SARS-CoV-2 vaccine was used in this study; 2 doses were administrated 4 weeks apart. In all, 16 patients (from the anti-CD38 group) received a third booster vaccine dose. The anti-CD38 group is defined as patients who were treated with anti-CD38 immunotherapy. The convalescent group is defined as patients with a history of SARS-CoV-2 infection who were given the vaccine. T0 is the time before vaccine, M1 is 1 month after the first vaccine dose, and M3 is 3 months after the first vaccine dose. (B-C) SARS-CoV-2–specific IgG and IgA production quantified by S-Flow in 60 patients with MM who had never been infected with SARS-CoV-2 and 23 healthy volunteers. (B) IgG (left panel) and IgA (right panel) quantification in controls or patients with MM before vaccination (T0) and at M1 and M3. (C) Comparison of IgG amounts in patients with MM who were treated or not treated with anti-CD38 immunotherapy. (D-E) Quantification of anti-SARS-CoV-2 nAbs against alpha or delta variants in controls (n = 23) or patients with MM (n = 60). (D) Comparison between controls and patients with MM. (E) Comparison between patients receiving or not receiving anti-CD38 immunotherapy. (F) Quantification of cellular immune response by S1 or S2 EliSpot (in spot forming units, SFU, per 106 CD3 cells) in controls (n = 21) or patients with MM (n = 26). Patients receiving anti-CD38 immunotherapies are indicated. Error bars represent standard error. *P < .05; **P < .01; ***P < .001; ****P < .0001. ns, not significant.
First, we focused our analysis on patients with MM who had no history of SARS-CoV-2 infection (n = 60) and observed that only 44% and 85% of them achieved IgG seroconversion at M1 and M3, respectively, compared with 100% among the control group (supplemental Figure 1A). Moreover, IgG and IgA titers were significantly lower at M1 in patients with MM and remained lower for IgA at M3 compared with titers in the control group (Figure 1B). Next, we observed lower IgG but similar IgA titers in patients with MM who received anti-CD38 treatment compared with those who received alternative treatments (Figure 1C; supplemental Figure 1B). To assess protection against SARS-CoV-2, we measured the production of nAbs against alpha and delta variants. nAb response was significantly higher against the alpha variant, but we observed a strong correlation between alpha and delta response across patients with MM (supplemental Figure 1C-D). Although all participants in the control group developed nAbs against both variants after vaccination, only 51% and 41% of patients with MM developed nAbs against alpha and delta variants, respectively, and alpha and delta nAb titers were significantly lower in patients with MM compared with those in controls (Figure 1D). Among patients with MM, anti-CD38 therapy further reduced nAb production against the alpha variant, with a near-significant trend for the delta variant (Figure 1E). In patients with MM, we observed a low correlation between anti-S IgG or IgA and anti-alpha or -delta nAb production, chiefly because patients developed IgG or IgA but not nAbs (supplemental Figure 1E).
We investigated the humoral response on day 21 in 16 patients with MM who were in the anti-CD38 group and who received a third dose of BNT162b2 at least 21 days after the second dose (booster protocol). We observed an improvement in the S response in half the patients who had achieved seroconversion to the second dose (Bethesda units [BU] >0.6) and a significant increase in IgG titers in patients with suboptimal response (BU <0.6) (supplemental Figure 2A). However, no significant improvement of alpha or delta nAb response was observed even if increased nAbs were observed in some patients (supplemental Figure 2B).
Finally, we observed that an absence of neutralizing response against alpha and delta variants after the second dose was significantly associated with progressive disease, number of treatment lines, and lower gammaglobulin levels, and that anti-CD38 therapy was associated with failure to generate nAbs (supplemental Table 2). However, none of these variables retained statistical significance after multivariable analysis, possibly because of the limited number of patients included in the study or the interactions between variables. Because patients with MM who were treated or not treated with anti-CD38 received immunomodulatory imide drugs or proteasome inhibitors in similar proportions, our results suggest that impaired immune response to SARS-CoV-2 vaccine is favored by targeting nonmalignant B cells, as reported in patients receiving anti-CD20 antibodies.6-8
We investigated anti–SARS-CoV-2 T-cell response after vaccination in 26 patients with MM from our cohort, including 24 who received anti-CD38 therapy and 21 healthy volunteers 3 months after the first dose of BNT162b2. The IFN-γ production by T cells after stimulation with S1 or S2 antigens ex vivo was significantly lower in patients with MM compared with that in healthy controls who had never been infected by SARS-CoV-2 (Figure 1F). Among 26 patients with MM tested for cellular response, 12 (46%) lacked nAbs and IFN-γ production, 5 (19%) developed both humoral and cellular response, and 9 (35%) showed heterogeneous results (supplemental Figure 3). Notably, all controls had both nAbs and specific T-cell response after vaccination with BNT162b2 (data not shown). These results show an altered cellular response to anti–SARS-CoV-2 vaccine in patients with MM.
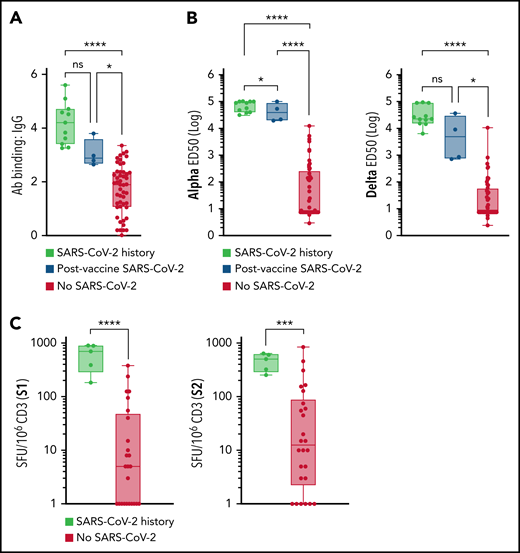
Next, we studied the immune response to BNT162b2 that depended on patients having a history of SARS-CoV-2 infection. First, we observed higher anti-S IgG levels in patients with MM who had a history of SARS-CoV-2 infection before (n = 11) or after (n = 4) vaccination compared with other patients, with no differences regarding the timing of infection or vaccination (Figure 2A). Similarly, patients with MM who were infected with SARS-CoV-2 had higher alpha- and delta-specific nAb levels and T-cell IFN-γ response compared with those who had not been infected (Figure 2B-C).
Impact of SARS-CoV-2 infection on immunologic response to BNT162b2 in patients with MM. (A-C) Patients with MM with a history of infection with SARS-CoV-2 (more than 3 months before vaccination) who developed their infection at any time after vaccination or who had never had SARS-CoV-2 infection. (A) S-Flow IgG quantification (history of SARS-CoV-2 infection, n = 11; post-vaccine SARS-CoV-2, n = 4; other, n = 56). (B) Alpha (left) or delta (right) nAb quantification (history of SARS-CoV-2 infection, n = 11; post-vaccine SARS-CoV-2, n = 4; other, n = 56). (C) S1 (left) or S2 (right) IFN-γ EliSpot (SARS-CoV-2 history, n = 5; other, n = 21). Error bars represent standard error. *P < .05; ***P < .001; ****P < .0001. ns, not significant.
Impact of SARS-CoV-2 infection on immunologic response to BNT162b2 in patients with MM. (A-C) Patients with MM with a history of infection with SARS-CoV-2 (more than 3 months before vaccination) who developed their infection at any time after vaccination or who had never had SARS-CoV-2 infection. (A) S-Flow IgG quantification (history of SARS-CoV-2 infection, n = 11; post-vaccine SARS-CoV-2, n = 4; other, n = 56). (B) Alpha (left) or delta (right) nAb quantification (history of SARS-CoV-2 infection, n = 11; post-vaccine SARS-CoV-2, n = 4; other, n = 56). (C) S1 (left) or S2 (right) IFN-γ EliSpot (SARS-CoV-2 history, n = 5; other, n = 21). Error bars represent standard error. *P < .05; ***P < .001; ****P < .0001. ns, not significant.
Among our cohort of 60 patients with MM who had never been infected with SARS-CoV-2, 4 developed an infection after 1 dose (n = 2) or 2 doses (n = 2) of BNT162b2, and all were receiving anti-CD38 immunotherapy (Figure 1A). We retrospectively recorded mortality related to SARS-CoV-2 from January 2020 to July 2021 in 39 public hospitals in Paris, France, using the Informatics for Integrated Biology and the Bedside (i2b2) platform. When comparing 2 epidemic peak periods (March to July 2020 and March to July 2021), the number of deaths related to SARS-CoV-2 infection remained stable or tended to decrease in patients with MM who were or were not receiving anti-CD38 immunotherapy, respectively, even though anti-CD38 prescription volume remained stable (supplemental Figure 4A-B). Although several biases could have occurred in this retrospective multisite analysis, these results along with our observations in a small cohort suggest that impaired vaccine response in patients receiving anti-CD38 could have clinical implications that should be investigated prospectively.
In contrast to observations made among healthy populations,3,9 correlation between the commonly used S-Flow antibody titration technique and the detection of nAbs was weak in patients with MM, suggesting a low predictive value of serology in terms of protection. Moreover, emergence of SARS-CoV-2 variants with lower response to vaccination, such as the delta variant is particularly problematic for individuals with suboptimal vaccine response.3,10 In agreement with recent reports, we observed heterogeneous humoral response to vaccine in patients with MM who had never been infected with SARS-CoV-2.11 Patients with MM who were treated with anti-CD38 immunotherapy remain at higher risk of SARS-CoV-2 infection after mRNA-based vaccination compared with patients who receive alternative therapies.12 In contrast, patients with MM who became infected with SARS-CoV-2 before or after vaccination had homogeneous and robust immune response. Our preliminary results on booster vaccination also suggest that immune response to SARS-CoV-2 remains actionable in patients with MM. Improvement of vaccine formulation such as adjuvant use or heterologous vaccination procedure13 should be considered in this frail population.
Acknowledgments
The authors thank the patients and the healthy volunteers who participated in this study and all nurses, technicians, and physicians from Groupe Hospitalo-Universitaire AP-HP.CUP involved in the daily care of the patients included or not included in this study; they have gone far beyond their usual jobs during this epidemic.
This study was supported by Fonds IMMUNOV for Innovation in Immunopathology, by the Institut Pasteur (for work in the OS laboratory), the Urgence COVID-19 Fundraising Campaign of Institut Pasteur, Fondation pour la Recherche Médicale, and ANRS, and by grants from the Vaccine Research Institute (ANR-10-LABX-77), Labex IBEID (ANR-10-LABX-62-IBEID), ANR/FRM Flash Covid PROTEO-SARS-CoV-2, and IDISCOVR. D.P. is supported by the Vaccine Research Institute.
Authorship
Contribution: S.H., J.Z., T.B., A.O., and D.P. designed the study, collected and analyzed data, and wrote the manuscript; T.B., A.O., D.P., and I.S. designed and performed experiments; P.D., J.H., B.V., N.E., D.B., L.W., G.F., J.D., P.F., and B.D.-F. provided care for the patients; B.T. and J.T. analyzed data and wrote the manuscript; L.C. and O.S. designed and supervised experiments and wrote the manuscript; and M.V. designed and supervised the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: T.B., I.S., and O.S. are co-inventors on a provisional patent (US 63/020 063) entitled “S-Flow: a FACS-based assay for serological analysis of SARS-CoV-2 infection” submitted by Institut Pasteur. The remaining authors declare no competing financial interests.
Correspondence: Marguerite Vignon, Department of Clinical Hematology, Hôpital Cochin, 27 Rue du Faubourg Saint-Jacques, 75014 Paris, France; e-mail: marguerite.vignon@aphp.fr.
For original data, please contact Soledad Henriquez via email at soledad.henriquez@aphp.fr.
The online version of this article contains a data supplement.
REFERENCES
Author notes
S.H., J.Z., T.B., and A.O. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal