In this issue of Blood, Yang et al1 show that HBO1 (KAT7) promotes hematopoietic stem cell (HSC) quiescence and self-renewal through histone acetylation and transcription of HSC genes, revealing an important role for HBO1 in maintaining the blood system.
Acetylation, one of the oldest described histone modifications, involves the transfer of an acetyl group from acetyl-CoA to the ɛ-amino group of a lysine residue by histone acetyltransferases.2 Histone acetylation acts to unfold chromatin to facilitate DNA access for replication or transcription. Histone H3 lysine 14 acetylation (H3K14ac) is found throughout gene bodies and promoters3 and promotes processivity of RNA polymerase. H3K14ac is mainly established by HBO1, a conserved, widely expressed member of the MYST acetyltransferase family.4 The MYST family includes 4 additional acetyltransferases: KAT6A, KAT6B, KAT8 (MOF), and KAT5 (TIP60). HBO1 acts as a core catalytic subunit in multimeric complexes along with a host of cofactors that includes MEAF6, ING4, ING5, BRPF1, BRPF2, BRPF3 (H3K14), JADE1, JADE2, and JADE3 (H4). HBO1 and its cofactors work in concert with other epigenetic regulators in various complexes, such as MLL, that promote epigenetic or transcriptional activation.5
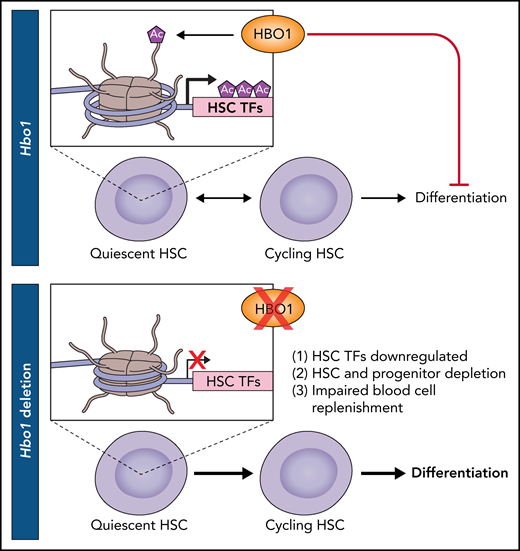
Yang et al provide important new insights into the role of HBO1 in normal HSC function and blood replenishment (see figure). The investigators produced 2 mouse models that enabled inducible deletion of exon 1 of Hbo1. Deletion led to HSC loss and significantly decreased production of blood cells of all lineages, suggesting HSC exhaustion as a potential cause. Further experimentation suggested that depletions were apparent in the HSC compartment before losses in the differentiated populations. The authors strengthened these findings through experiments with competitive transplantation into lethally irradiated mice. Hbo1fl/+;CreER (heterozygous) donor cells contributed 56% to 86% of peripheral blood cells in bone marrow chimeras whereas Hbo1fl/fl;CreER (deletion) donor cells made no detectable contribution. Furthermore, lower proportions of HSCs and multipotent progenitors were found in G0 and approximately twofold more were found in S and G2-M, indicating a depletion of the HSC quiescent state, which is often linked to loss of self-renewal.6 Indeed, the fate of HSCs with deletion of Hbo1 was shifted toward differentiation at the expense of symmetric self-renewal, indicating that Hbo1 is required for HSC self-renewal. Mechanistically, H3K14ac CUT&Tag experiments in Hbo1-deleted HSCs revealed a widespread loss of this epigenetic mark across gene bodies, promoters, and downstream of transcription end sites. In Lin–Sca1+Kit+ cells with Hbo1 deletion, strong reductions in H3K14ac were found at canonical HSC self-renewal genes. These genes, including Hoxa9, Meis1, and Erg, were consequently downregulated. The comprehensive analysis of hematopoiesis in the absence of HBO1 thus yields a coherent picture of H3K14ac loss and downregulation of HSC genes, increased stem cell proliferation and differentiation, and reduced replenishment of mature blood cells.
Model of HSC quiescence and self-renewal promoted by HBO1-mediated H3K14 acetylation (Ac). Deletion of Hbo1 results in downregulation of transcription factors (TFs) that are important for HSC maintenance, leading to increased differentiation. Professional illustration by Patrick Lane, ScEYEnce Studios.
Model of HSC quiescence and self-renewal promoted by HBO1-mediated H3K14 acetylation (Ac). Deletion of Hbo1 results in downregulation of transcription factors (TFs) that are important for HSC maintenance, leading to increased differentiation. Professional illustration by Patrick Lane, ScEYEnce Studios.
Given that histone acetylation maintains cellular homeostasis, acetyltransferases play complex and differential roles in disease. Germline mutations in acetyltransferases lead to severe developmental defects, and mutations in adults can lead to malignancy.5 Translocations and mutations in CBP, p300, TIP60, KAT6A, and KAT6B are often found in acute myeloid and lymphoid leukemias, as well as numerous solid tumors. Unsurprisingly, inhibitors to acetyltransferases have been developed. For example, KAT6A and KAT6B inhibitors arrest lymphoma progression by inducing senescence, CBP and p300 inhibition can relieve oncogene-induced differentiation block in leukemia, and TIP60 inhibitors induce apoptosis of breast cancer cells.5,7 Various inhibitors of acetyltransferase family members have been discovered but relatively few have entered clinical trials. Recent studies put forth an HBO1 inhibitor candidate, WM-3538, which broadly inhibits MYST family members as a competitive analog of acetyl-CoA.8 Acetyltransferase inhibition has diverse effects in different cell types, complicating their suitability for therapeutic treatment. For example, genetic targeting has revealed that HBO1 has important functions in promoting senescence,9 survival of MLL-rearranged leukemia stem cells,8,10 and now maintenance of normal HSCs. These various effects should be taken into account when designing therapeutic regimens.
The role of histone acetyltransferases such as HBO1 in regulating cell fate and self-renewal highlights the importance of understanding the functions of the acetyltransferase family of epigenetic enzymes. The fundamental knowledge established by the work of Yang et al will inspire the development of new epigenetic modulators for therapeutic purposes.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal