Key Points
Loss of CCR4 expression is common after treatment of CTCL with mogamulizumab.
Mutations and deletions of CCR4 emerge in a subset of patients after mogamulizumab treatment.
Abstract
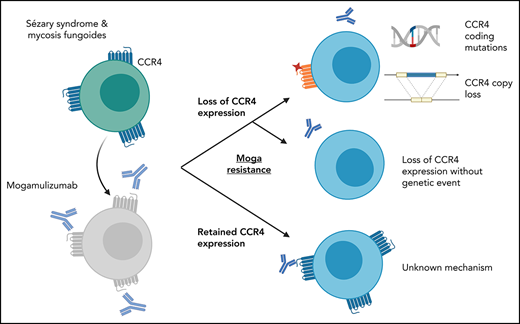
Mogamulizumab is a humanized anti–CC chemokine receptor 4 (CCR4) antibody approved for the treatment of mycosis fungoides and Sézary syndrome. Despite almost universal expression of CCR4 in these diseases, most patients eventually develop resistance to mogamulizumab. We tested whether resistance to mogamulizumab is associated with loss of CCR4 expression. We identified 17 patients with mycosis fungoides or Sézary syndrome who either were intrinsically resistant or acquired resistance to mogamulizumab. Low expression of CCR4 by immunohistochemistry or flow cytometry was found in 65% of patients. Novel emergent CCR4 mutations targeting the N-terminal and transmembrane domains were found in 3 patients after disease progression. Emerging CCR4 copy number loss was detected in 2 patients with CCR4 mutations. Acquisition of CCR4 genomic alterations corresponded with loss of CCR4 antigen expression. We also report on outcomes of 3 cutaneous T-cell lymphoma (CTCL) patients with gain-of-function CCR4 mutations treated with mogamulizumab. Our study indicates that resistance to mogamulizumab in CTCL frequently involves loss of CCR4 expression and emergence of CCR4 genomic alterations. This finding has implications for management and monitoring of CTCL patients on mogamulizumab and development of future CCR4-directed therapies.
Introduction
CC chemokine receptor 4 (CCR4) has emerged as an effective therapeutic target for cutaneous T-cell lymphomas (CTCL) and has been shown to have almost universal expression in Sézary syndrome and mycosis fungoides.1,2 Mogamulizumab is a defucosylated, humanized anti-CCR4 monoclonal antibody that binds to the N-terminal domain of CCR4 with high affinity and promotes antibody-dependent cytotoxicity of the malignant T cells.2 A randomized phase 3 trial demonstrated superior outcomes with mogamulizumab as compared with vorinostat, including improved overall response rate and improved median progression-free survival.3 However, many patients, including those with an initial complete response, may ultimately develop resistance to the drug, and only 11% of patients will have a response lasting ≥12 months.4 Mechanisms of resistance to mogamulizumab have not been elucidated. Here, we report that loss of CCR4 expression and genomic alterations in the CCR4 gene are recurrent mechanisms of resistance to mogamulizumab in CTCL.
Study design
We identified patients with CTCL treated with mogamulizumab at Stanford’s Cutaneous Lymphoma Clinic between October 2009 and March 2021 who had discontinued treatment due to lack or loss of response. Primary mogamulizumab resistance was defined as lack of global response to treatment at any timepoint, whereas secondary resistance was defined as relapse after an initial global response of any duration per consensus criteria.5 Archival blood and skin and/or lymph node biopsies after the time of progression were studied. Specimens collected prior to initiation of mogamulizumab were also procured whenever available. Additionally, we identified 3 patients with CTCL treated with mogamulizumab who had C-terminal gain-of-function (GoF) CCR4 mutations6,7 identified by the HemeSTAMP targeted sequencing panel.8 This study was approved by the Institutional Review Board at Stanford University.
CCR4 protein expression was determined by immunohistochemistry (clone L291H4; Biolegend) and by flow cytometry in patients with circulating Sézary cells and in transfected Jurkat cells (clone KM2160; Kyowa Medical Corporation, and clone IG1; BD Biosciences). For flow cytometry, the mean fluorescence intensity (MFI) was calculated as the MFI of the CCR4 antibody minus the MFI of an isotype control. Testing of samples collected during mogamulizumab treatment verified that treatment with mogamulizumab did not interfere with immunohistochemistry (IHC) detection of CCR4. Tumor or germline DNA was purified from formalin-fixed, paraffin-embedded sections or peripheral blood mononuclear cells as previously described.9 Targeted sequencing was performed with a custom Agilent SureSelect panel that included full coverage of the exonic regions of CCR4. All CCR4 variants were manually reviewed using the Integrative Genomics Viewer in all available specimens from the same patient. Copy number changes were assessed by CNVkit.10 A pcDNA3.1 vector with wild-type or variant CCR4 complementary DNA with a DYK tag (GenScript) was transfected into Jurkat cells using a Cell Line Nucleofector Kit (Lonza) and selected with G418. Antibody-dependent cellular cytotoxicity assays were performed with anti-CCR4 human clone KW0761 (Novus) using a reporter assay with Jurkat cells expressing the FcγRIIIa receptor and firefly luciferase under control of an NFAT response element (Promega). The Jurkat reporter cells were coincubated overnight with Jurkat cells transfected with wild-type or mutant CCR4 at a ratio of 1:1.
Results and discussion
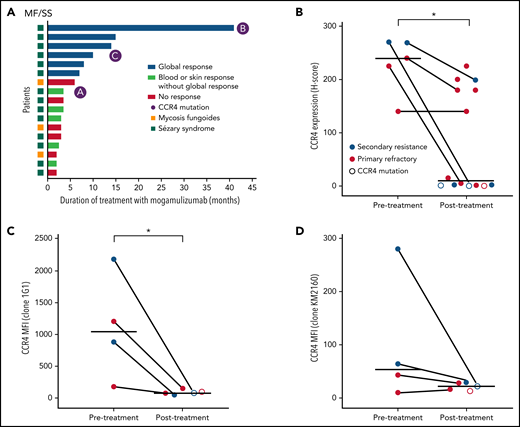
We identified 17 patients with evaluable samples who had progressed after mogamulizumab treatment. Eleven patients were classified as primary refractory to treatment, and the remaining 6 patients had secondary resistance. Nodal or visceral involvement was present in 6 patients, and 4 patients had histologic evidence of large cell transformation at the time of treatment with mogamulizumab. Median duration of treatment with mogamulizumab was 3.5 months (range, 2-41) (Figure 1A).
CCR4 expression decreases in a subset of patients after mogamulizumab treatment. (A) Total duration of treatment with mogamulizumab and best treatment responses. (B) CCR4 IHC expression pre- and posttreatment with mogamulizumab. Bars indicate mean H score. Lines connect paired samples from the same patient. (C-D) MFI of CCR4 by flow cytometry before and after treatment using 2 CCR4 antibodies: 1G1 (C) and KM2160 (D). *P < .05 by Mann-Whitney U test. H score, percentage of the CCR4+ cells among all cells in the infiltrate x intensity of expression 0-3+.
CCR4 expression decreases in a subset of patients after mogamulizumab treatment. (A) Total duration of treatment with mogamulizumab and best treatment responses. (B) CCR4 IHC expression pre- and posttreatment with mogamulizumab. Bars indicate mean H score. Lines connect paired samples from the same patient. (C-D) MFI of CCR4 by flow cytometry before and after treatment using 2 CCR4 antibodies: 1G1 (C) and KM2160 (D). *P < .05 by Mann-Whitney U test. H score, percentage of the CCR4+ cells among all cells in the infiltrate x intensity of expression 0-3+.
CCR4 expression was absent by immunohistochemistry in posttreatment specimens of 8 of 14 patients studied (Figure 1B). This included patients who were primary refractory and those who developed secondary resistance to mogamulizumab. In 2 cases, biopsies collected prior to mogamulizumab treatment confirmed intact CCR4 expression before therapy. Flow cytometry showed similar results with decreased or absent expression of CCR4 in patients with prior treatment with mogamulizumab (Figure 1C-D). One refractory patient had low CCR4 expression prior to treatment. The loss of CCR4 expression was not associated with any clear change in the clinical course, nor was it associated with any phenotypic change, such as the development of large cell transformation.
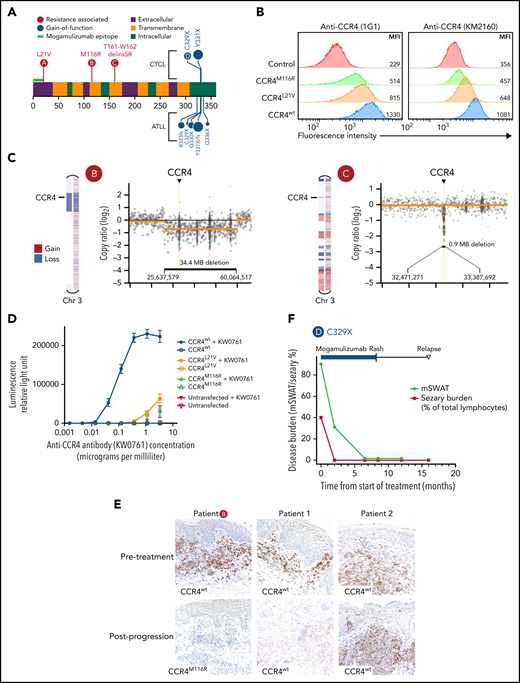
Targeted DNA sequencing revealed novel coding mutations of CCR4 in 3 patients with Sézary syndrome (Figure 2A). In all 3 cases, the mutations were not detectable prior to mogamulizumab treatment. These mutations localized to the N-terminal half of CCR4 and are distinct from the previously described C-terminal GoF CCR4 mutations in CTCL and adult T-cell leukemia/lymphoma.6,7 Only the CCR4L21V variant overlapped with the known N-terminal mogamulizumab binding epitope. The other 2 mutations occurred in transmembrane domains (Figure 2A). Transfection of the CCR4L21V and CCR4M116R variants into Jurkat cells resulted in decreased CCR4 expression as compared with CCR4wt (Figure 2B). Two patients with mutations in CCR4 also demonstrated copy number loss involving the CCR4 locus (Figure 2C), suggesting deletion of the wild-type CCR4 allele. CCR4 genomic alterations were associated with loss of CCR4 expression in all 3 patients. Although these N-terminal and transmembrane CCR4 mutations retained partial expression and binding affinity for mogamulizumab based on flow cytometry, both CCR4L21V and CCR4M116R mutations resulted in markedly impaired antibody-dependent cellular cytotoxicity (Figure 2D).
Mutations in CCR4 arising after mogamulizumab treatment result in decreased CCR4 expression and reduced antibody dependent cellular cytotoxicity. (A) Diagram of resistance-associated CCR4 mutations (red circles) in 3 mogamulizumab-treated patients in relation to the mogamulizumab-binding epitope, transmembrane domains, and previously described GoF mutations (blue circles). (B) Jurkat cells were transfected with either CCR4wt, CCR4M116R, or CCR4L21V. MFI of CCR4 by flow cytometry is shown using 2 CCR4 antibodies: 1G1 (left) and KM2160 (right). (C) Normalized copy ratio of reads binned across chromosome 3. Yellow line indicates segmented copy number alterations. (D) Antibody-dependent cellular cytotoxicity assay. Jurkat transfectants were cocultured with a Jurkat reporter cell line expressing FcγRIIIa and firefly luciferase under an NFAT response element. Cells were incubated overnight with or without the human anti-CCR4 antibody KM0761 prior to detection of luciferase activity. Erros bars indicate standard deviation. (E) IHC staining of CCR4 in skin biopsies of 3 patients before (top) and after (both) progression on mogamulizumab. Each row of images represents paired biopsies from the same patient. (F) Response to mogamulizumab in the skin (black) and blood (red) in a patient with Sézary syndrome and a C329X GoF mutation of CCR4.
Mutations in CCR4 arising after mogamulizumab treatment result in decreased CCR4 expression and reduced antibody dependent cellular cytotoxicity. (A) Diagram of resistance-associated CCR4 mutations (red circles) in 3 mogamulizumab-treated patients in relation to the mogamulizumab-binding epitope, transmembrane domains, and previously described GoF mutations (blue circles). (B) Jurkat cells were transfected with either CCR4wt, CCR4M116R, or CCR4L21V. MFI of CCR4 by flow cytometry is shown using 2 CCR4 antibodies: 1G1 (left) and KM2160 (right). (C) Normalized copy ratio of reads binned across chromosome 3. Yellow line indicates segmented copy number alterations. (D) Antibody-dependent cellular cytotoxicity assay. Jurkat transfectants were cocultured with a Jurkat reporter cell line expressing FcγRIIIa and firefly luciferase under an NFAT response element. Cells were incubated overnight with or without the human anti-CCR4 antibody KM0761 prior to detection of luciferase activity. Erros bars indicate standard deviation. (E) IHC staining of CCR4 in skin biopsies of 3 patients before (top) and after (both) progression on mogamulizumab. Each row of images represents paired biopsies from the same patient. (F) Response to mogamulizumab in the skin (black) and blood (red) in a patient with Sézary syndrome and a C329X GoF mutation of CCR4.
Thus, we find that patients with resistance to mogamulizumab fall into 3 categories: (1) CCR4 antigen loss associated with genomic events disrupting CCR4 (Figure 2E, left), (2) loss of CCR4 expression in the absence of detectable genomic events (Figure 2E, middle), and (3) an as-of-yet undetermined mechanism of mogamulizumab resistance with retained high CCR4 expression (Figure 2E, right). Low CCR4 expression was identified in 10 of 14 patients with Sézary syndrome and 1 of 3 patients with mycosis fungoides. Additional studies of mycosis fungoides are needed to determine if CCR4 loss is common among mogamulizumab-treated patients. These findings have practical implications for the development and use of the next generation of CCR4-targeting therapies, such as cellular therapies currently in development. Our results suggest that CCR4-targeted therapies may only be effective in less than half of patients previously treated with mogamulizumab and that screening for CCR4 expression should be considered to select patients more likely to respond to second-line therapies.
C-terminal GoF CCR4 mutations are associated with a higher response rate and improved survival in ATLL patients treated with mogamulizumab.11,12 We identified 3 CTCL patients who harbored these types of classic GoF CCR4 mutations and were treated with mogamulizumab. One patient with Sézary syndrome with a C329X mutation obtained a durable near-complete response to mogamulizumab treatment (Figure 2F). Her response persisted 8 months after interruption of mogamulizumab treatment due to a drug-associated rash. Two patients with mycosis fungoides with large cell transformation and a CCR4 Y331X mutation were also treated with mogamulizumab without clinical response. Therefore, presence of a GoF CCR4 mutation in CTCL does not consistently predict a favorable response to mogamulizumab, and features such as CTCL subtype and presence of large cell transformation may be important considerations in selection of CCR4 therapy for patients with a CCR4 GoF mutation.
Overall, this work suggests that loss of CCR4 expression under the selective pressure of CCR4 therapies may represent a major hurdle in the development of future CCR4-targeted strategies in CTCL.
Acknowledgments
The authors acknowledge Ronald Levy and Shoshana Levy for their helpful suggestions and review.
This work was supported, in part, by research from National Institutes of Health, National Cancer Institute grant K08 CA207882-01 (M.S.K.), the American Society of Hematology Research Training Award for Fellows (S.B.), and the Haas Family Foundation (Y.H.K. and M.S.K.). Graphical abstract created with Biorender.com.
Authorship
Contribution: S.B., G.E.D., S.F.-P., and M.S.K. designed and performed experiments; A.H.R., Y.H.K., and M.S.K. provided study material; all authors analyzed data and interpreted results; and S.B. and M.S.K. wrote the manuscript with input from all authors.
Conflict-of-interest disclosure: S.B. is an employee and has stock ownership of Kite Pharma. Y.H.K. has received research funding from Kyowa Kirin, Innate Pharma, Corvus Pharmaceuticals, CRISPR Therapeutics, Trillium Therapeutics, and reports advisory board participation for Kyowa Kirin, Galderma Pharmaceuticals, Mundipharma, and Secura Bio. M.S.K. has received research funding from CRISPR Therapeutics and Nutcracker Therapeutics and consulting fees from Myeloid Therapeutics and Daiichi Sankyo. The remaining authors declare no competing financial interests.
Correspondence: Michael S. Khodadoust, Division of Oncology, Department of Medicine, Stanford University, 1701 Page Mill Rd, Palo Alto, CA 94304; e-mail: mkhodado@stanford.edu.
Send data sharing requests via e-mail to the corresponding author.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
S.B. and G.E.D. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal