Abstract
More than 100 years ago, Duke transfused whole blood to a patient with thrombocytopenia to raise the platelet count and prevent bleeding. Since then, platelet transfusions have undergone numerous modifications from whole blood–derived platelet-rich plasma to apheresis-derived platelet concentrates. The storage time and temperature have also changed. The mandate to store platelets for a maximum of 5 to 7 days at room temperature has been challenged by recent clinical trial data, ongoing difficulties with transfusion-transmitted infections, and recurring periods of shortages that were further exacerbated by the COVID-19 pandemic. Alternative platelet storage approaches are as old as the first platelet transfusions. Cold-stored platelets may offer increased storage times (days) and improved hemostatic potential at the expense of reduced circulation time. Frozen (cryopreserved) platelets extend the storage time to years but require storage at −80°C and thawing before transfusion. Lyophilized platelets can be powder-stored for years at room temperature and reconstituted within minutes in sterile water but are probably the least explored alternative platelet product to date. Finally, whole blood offers the hemostatic spectrum of all blood components but has challenges such as ABO incompatibility. We know more than ever before about the in vitro properties of these products, and clinical trial data are accumulating. The purpose of this review is to summarize the findings of recent preclinical and clinical studies on alternative, donor-derived platelet products.
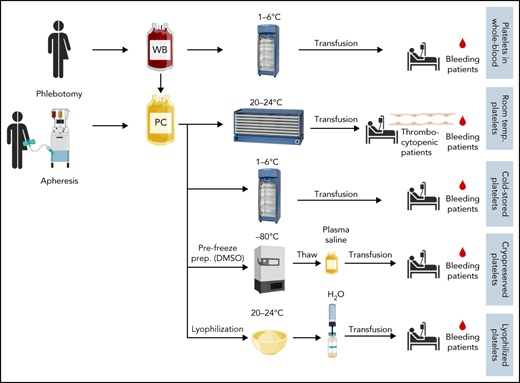
Introduction
Approximately 2.5 million platelet doses are administered annually in the United States to prevent (prophylactic transfusions) or treat bleeding (therapeutic transfusions). Platelets are stored at room temperature (RT; 20°C-24°C) in gas permeable storage bags with continuous agitation. RT storage maximizes circulation time but causes platelet dysfunction summarized as “storage lesion.” RT storage also permits bacterial growth, leading to a maximum storage time of 5 to 7 days to reduce the risk of septic transfusion reactions. In contrast, red blood cells (RBCs) are stored for up to 42 days (with additive solutions) at 1°C to 6°C. The short storage duration for platelets leads to frequent shortages, outdates, and wastage.1 In contrast to the decrease in RBC transfusions, use of platelets in the United States increased by 15.8% from 2017 to 2019.2 During that time, the number of distributed platelets decreased by 2%, which highlights a worrisome trend. The supply is especially challenging in rural areas and far forward military facilities. The number of available platelet units for transfusion fluctuates because of a tenuous inventory, and the ongoing COVID-19 pandemic further exacerbates these shortages.3 Ultimately, short storage time, septic transfusion reactions, and reduced function are caused by RT storage. In recently revised guidance, the US Food and Drug Administration (FDA) increased the requirements for bacterial testing needed to limit bacterial growth and enhance the detection of bacterially contaminated units. The new guidance will likely improve the safety of platelet transfusions, but it comes at the expense of increased costs, additional labor, and potential wastage of platelet units.4,5 In addition to septic reactions, RT storage of platelets likely contributes to the occurrence of other transfusion reactions, as the data from a small, retrospective study from 1977 suggest.6 One potential mechanism is the increased accumulation of cytokines at RT, which contributes to febrile nonhemolytic transfusion reactions.7
The historical desire to optimize stored platelets for thrombocytopenic patients led to an emphasis on maximizing circulation time.8 The largest group of platelet recipients is hematology-oncology patients, usually between 34% to 67% of the total patient population (depending on the study), and the majority of transfusions in this group are given prophylactically.9-13
Alternative platelet preparations, including cold-stored, lyophilized, and cryopreserved platelets (CPPs), have been investigated since platelet transfusions were first performed. Importantly, these alternative products are not considered “platelet products” by the FDA but are instead considered hemostatic products indicated for bleeding patients. The first platelets for transfusions were stored at cold temperatures.14 Freezing or freeze-drying (lyophilizing) platelets are also concepts developed over the last 5 to 6 decades.15-17 However, increasing appreciation and awareness of the limitations of RT-stored platelets have spawned a more widespread interest in this matter.
Besides helping bleeding patients, using alternative platelet products could also improve care for patients needing prophylactic transfusions. Diversifying the supply of platelet products will increase the availability of RT-stored inventory for prophylactic transfusions. A platelet count of 10 000/µL has been widely accepted as the trigger for prophylactic transfusions in patients with hypoproliferative thrombocytopenia.18 However, in the PLADO trial, bleeding occurred regardless of a platelet count between 6000 and 100 000/µL.19,20 Thus, there is a need for novel bleeding risk parameters other than the platelet count to assess endothelial health and integrity in patients.21,22 Viscoelastic testing (eg, thromboelastography) has been proposed as one such modality for predicting platelet function and guide therapy. Although its use resulted in reductions in RBC, platelet, and plasma transfusions, its effects on mortality and other clinically relevant parameters are inconsistent in various meta-analyses.23
At this time, alternative platelet products discussed in this review are considered only for therapeutic transfusions in bleeding patients. Just as the platelet count has limitations in predicting bleeding, RT-stored platelet transfusions have limitations in preventing bleeding. In the TOPPS trial, 43% of patients with hypoproliferative thrombocytopenia showed signs of bleeding despite receiving support with a prophylactic platelet transfusion.21 Alternative platelet products could promote endothelial integrity equal to or better than that of RT-stored platelets independently of the platelet count. Therefore, it is worth considering whether other indications for alternative platelet products could be tested in clinical trials.24-26 More importantly, although RT-stored platelets were long regarded as beneficial, or at least benign, recent clinical trial data show harmful effects in different patient populations.27,28 RT-stored platelet transfusions are also associated with increased risk for thrombosis and mortality in patients with thrombotic thrombocytopenic purpura and heparin-induced thrombocytopenia.29
Cold-stored platelets
Platelets were historically refrigerated for transfusion until the 1970s, when Murphy and Gardner8 showed decreased circulation half-life of cold-stored platelets (CSPs) compared with those stored at RT. However, there has been a renewed interest in investigating platelets held at 1°C to 6°C, given the lower risk of bacterial growth, potentially longer shelf life, and better in vitro function.30-32 Most of these studies tested platelets in their respective storage supernatant and disregarded the high degree of dilution that stored platelets undergo upon transfusion into recipients. Another advantage of CSPs is an earlier release for transfusion (∼24 hours after collection) because bacterial testing can be omitted; tests for donor infectious diseases are the only tests required.
Platelets undergo numerous structural, metabolic, and molecular changes during refrigeration (Figure 1). Platelets change from a normal discoid shape to a spherical shape that is associated with the loss of the circumferential microtubule ring and cytoskeletal rearrangement.33 Several studies showed successful pharmacologic inhibition of cold-induced shape change, albeit without any advantage for in vivo function.33-35 The effect of cold on platelets resembles the effect of common agonists, including an intracellular calcium increase. RT exposure alone elicits some of these changes (Figure 1).33,34,36-39 Leakage from intracellular calcium stores or reduced calcium exchanger activities at reduced temperatures are possible explanations; however, the exact mechanism is unknown. Mitochondrial function is better preserved, and the generation of basal reactive oxygen species (ROS) is lower in CSPs than in RT-stored platelets.40,41 ROS contributes to clearance, and the addition of N-acetylcysteine improved circulation time and function in CSPs.42
Refrigeration-induced phenotypic and functional changes in platelets associated with clearance. (A) Platelets circulate at 37°C (body core temperature). (B) Exposure to RT results in low-level activation with intracellular calcium release and integrin activation. (C) Further decreasing the temperature to 1°C to 6°C leads to shape change, increase in calcium concentration, and specific clearance mechanisms via αMβ1 on liver macrophages and VWF. (D) Extended storage leads to aggregate formation, clearance by the hepatic Ashwell-Morell receptor (AMR), loss of GPVI and other glycoproteins, phosphatidylserine (PS) exposure, and α-degranulation. MP, microparticles. Figure 1 was created at BioRender.com.
Refrigeration-induced phenotypic and functional changes in platelets associated with clearance. (A) Platelets circulate at 37°C (body core temperature). (B) Exposure to RT results in low-level activation with intracellular calcium release and integrin activation. (C) Further decreasing the temperature to 1°C to 6°C leads to shape change, increase in calcium concentration, and specific clearance mechanisms via αMβ1 on liver macrophages and VWF. (D) Extended storage leads to aggregate formation, clearance by the hepatic Ashwell-Morell receptor (AMR), loss of GPVI and other glycoproteins, phosphatidylserine (PS) exposure, and α-degranulation. MP, microparticles. Figure 1 was created at BioRender.com.
Other changes include slower glycolytic metabolism, reduced cytokine release,43 increased expression of activation markers (P-selectin, phosphatidylserine, activated integrin αIIbβ3),41 and greater generation of platelet microparticles.41 CSPs have a greater capacity to reduce endothelial cell permeability than RT-stored platelets in vitro. This effect was reversed in vivo, likely because of the reduced circulation time of CSPs.24
Normalizing the circulation time of CSPs is a holy grail in transfusion medicine. One could speculate that platelets are cleared quickly because of cold-induced damage or a safety mechanism to remove hyperfunctional platelets. Lowering the storage temperature marginally (eg, to 18°C) markedly shortens the circulation time.44 Murine data suggest that the clearance is independent of shape change.45 The cold-induced GPIb-V-IX clusters facilitate the binding of GPIbα to von Willibrand factor (VWF).46,47 The survival of CSPs increased in VWF-deficient mice or with inhibition of platelet GPIbα-VWF interaction, suggesting a VWF-dependent clearance mechanism.46,48,49 The study by Josefsson et al50 demonstrated that short-term cold storage induces GPIbα clustering with βGlcNAc exposure. Although galactosylation of βGlcNAc did improve the survival of CSPs in a mouse study, this was not observed in humans who received platelets stored for 48 hours.51,52 Further research into glycans during platelet storage revealed that prolonged storage (≥48 hours) exposes galactose. Galactose is recognized by the hepatic Ashwell-Morell receptor, which mediates CSP clearance. These data provide a mechanistic explanation for the failed human trial.47,53 Alternating between 4°C and 37°C during storage (thermocycling) promises to undo reversible changes and prevent irreversible changes of cold storage while increasing circulation time.54 This approach improves platelet survival but does not normalize it.55 Taken together, accelerated clearance of CSPs is likely multifactorial and follows specific kinetics. Our current knowledge is mainly based on murine and cell culture data, and thus far, no approach has prevented decreased circulation time in humans.
During the 1960 and 1970s, multiple groups transfused CSPs to humans on acetylsalicylic acid and patients with thrombocytopenia and measured bleeding time and platelet counts. The data regarding efficacy are conflicting.56-59 Becker et al58 initially reported shorter bleeding times with CSPs but later acknowledged that the storage concentration of the control RT product was too high.57,58 The numerous changes in blood banking practices over the last few decades, the use of the bleeding time, and the short storage times used (≤48 hours) make it difficult to interpret these studies.
More recent radiolabeling studies show that CSPs undergo a continuous decline in platelet recovery from 5 to 20 days of storage, with a low nadir after day 10.41 The in vivo platelet recoveries are higher with plasma than with platelet additive solutions (PASs) for extended-stored CSPs.60 This could be important because PASs prevented platelet count decrease and aggregate formation in CSPs.61 However, some studies reported decreased platelet counts despite the use of PASs.62-64 Micro- and macroaggregates in CSPs form presumably because of interaction of plasma proteins with activated platelet receptors.61 Routinely used bedside filters decrease the number of transfused aggregates.65 Unsurprisingly, macroaggregates (ie, visible aggregates) are associated with wastage of CSPs.66 PASs could also play a role for CSPs beyond aggregate prevention. In a recent, randomized cross-over study, CSPs were as efficacious as RT-stored platelets in reversing the effect of aspirin 1 hour after transfusion. Notably, the platelet response to collagen was significantly better in recipients of RT-stored platelets after transfusion, likely because of reduced collagen receptor (glycoprotein VI) levels on CSPs.67 One in vitro study suggests that receptor levels in CSPs stored with PASs were not significantly different from those in RT-stored platelets.68
A pilot trial from Norway explored the safety and efficacy of transfusing CSPs stored for 7 to 14 days to patients undergoing cardiothoracic surgery. No significant differences in the median chest drain output were observed between patients transfused with CSPs and those transfused with RT-stored platelets.69 These limited data need confirmation in larger, randomized controlled trials. Several clinical trials are in progress or beginning soon (CHIPS [NCT 04834414]; CHASE [NCT05220787]), patients in hemorrhagic shock (CriSP-HS [NCT04667468]), and patients with traumatic brain injury (CriSP-TBI [NCT 04726410]). Institutions can now apply to the FDA for a variance to use CSPs stored for 14 days. Use is limited to bleeding patients if no RT-stored platelets are available or practical. CSPs are not currently indicated for prophylactic transfusions. The U.S. military and a civilian blood center received approval to produce CSPs stored for 14 days in early 2020.70 The original 3-day variance included only whole blood–derived platelets, based on the historical usage of this product, but it was extended to apheresis platelets.66 Although historical and preclinical studies used whole blood–derived platelets (eg, Acrodose platelets),42 and large parts of our knowledge about CSPs are based on this product, it is not currently included in variances and ongoing clinical trials.
CSPs in whole blood
Whole blood contains all cellular and soluble blood components, including platelets, RBCs, and plasma. Blood centers routinely collect whole blood and separate it into components for targeted transfusion therapy. Most transfusion services attempt to transfuse platelets in an ABO-identical fashion to maximize count increments. Although ABO-incompatible platelet transfusions were long considered benign, results from recent studies suggest that they worsen outcomes in patients with intracranial hemorrhage,71 and avoiding ABO-incompatible transfusions reduces the rate of alloimmunization.72 In addition to immunomodulatory effects, minor ABO incompatibility can result in rare, but catastrophic sequelae.73 Still, ABO-incompatible platelets are frequently transfused because of a lack of suitable inventory.
When transfusing whole blood as an emergency product, adhering to ABO identical products is not feasible because an inventory for all ABO types is impossible to maintain, and because the ABO type of bleeding patients is frequently unknown, especially outside the hospital. To avoid dangerous major ABO-incompatible whole blood transfusions, low-titer group O whole blood (LTOWB), which contains universally compatible RBCs and has low anti-A and anti-B titers is transfused to all blood types.74 Because group O is the most common ABO type in most populations, the majority of recipients will be ABO identical by chance. Nevertheless, minor ABO incompatibility will occur in A, B, and AB recipients.
Whole blood as a transfusion product has recently regained interest as a promising alternative in the prehospital emergency transfusion setting. Recent studies highlight the benefit of replacing crystalloids with early transfusions of blood products.75-77 Instead of transfusing multiple diluted products, providers need to transfuse only 1 product, which simplifies logistics and reduces donor exposure.78,79 Transfusing rhesus D antigen positive (or RhD+) whole blood to RhD– recipients is a concern, especially for females of childbearing age, although the relevance of this in a life-threatening massive transfusion scenario is debatable.80 Most cellular blood components in the United States are leukoreduced by filtering before storage to reduce alloimmunization and febrile nonhemolytic transfusion reactions. Whole blood can be leukoreduced with a platelet-sparing filter. The current storage maximum for whole blood is 21 days in citrate-phosphate-dextrose (CPD) and 35 days in citrate-phosphate-dextrose-adenine (CPDA-1) at 1°C to 6°C (Table 1).81
Overview of current and alternative platelet products
| Product . | Donors . | Collection . | Modifications . | Storage . | Storage duration . | (Planned) indication . | Bacterial growth risk . | Volume (mL) . | Preparation . | Summary . |
|---|---|---|---|---|---|---|---|---|---|---|
| RT-stored platelets (standard of care) | Regular donor restrictions | Apheresis/phlebotomy | Pathogen reduction, leukoreduction, irradiation, additive solutions, volume reduction | Liquid 20° to 24°C | 5-7 d | Prophylactic/therapeutic (current standard of care) | High (∼1:1000-3000)141 | 200-250 | N/A | Pro: Best in vivo recovery and survival Con: Short shelf life, bacterial growth, low in vitro quality |
| CSPs | Regular donor restrictions | Apheresis/phlebotomy | Additive solutions, leukoreduction | Liquid 1° to 6°C | 3 d, 14 d* | Therapeutic | Low143,144 | 200-250 | N/A | Pro: Extended shelf life, preserved in vitro quality Con: reduced in vivo recovery and survival, in vivo hemostatic function uncertain? |
| Cold-stored whole blood | Group O, low titer anti-A/B, possibly males only | Phlebotomy | Leukoreduction | Liquid 1°C to 6°C | 21 d (CPD), 35 d (CPDA-1) | Therapeutic (especially outside of hospital, trauma) | Low142 | 500 | N/A | Pro: Extended shelf life, includes all blood components Con: Possible ABO, Rh mismatch, lower recovery and survival, lower platelet dose, in vivo platelet function uncertain |
| CPPs | Regular donor restrictions | Apheresis/phlebotomy | Leukoreduction, resuspension in saline or plasma | Solid/frozen, −80°C | 3 y (≥5 y reported) | Therapeutic | Perceived to be very low16 | 20-50 | Thawing and resuspension† | Pro: Extended shelf life (years), almost normal in vivo survival, most in vivo performance data Con: logistics (freezer, thawing, preparation) |
| Freeze-dried (lyophilized) platelets‡ | Group O | Apheresis | Leukoreduction | Solid/powder, RT | 3 y | Therapeutic (especially outside of hospital, military) | Perceived to be very low (including pathogen reduction and culture)134 | 30-50 | Reconstitution in sterile water | Pro: Extended shelf life (years), logistics Con: reduced in vitro function, in vivo hemostatic function uncertain |
| Product . | Donors . | Collection . | Modifications . | Storage . | Storage duration . | (Planned) indication . | Bacterial growth risk . | Volume (mL) . | Preparation . | Summary . |
|---|---|---|---|---|---|---|---|---|---|---|
| RT-stored platelets (standard of care) | Regular donor restrictions | Apheresis/phlebotomy | Pathogen reduction, leukoreduction, irradiation, additive solutions, volume reduction | Liquid 20° to 24°C | 5-7 d | Prophylactic/therapeutic (current standard of care) | High (∼1:1000-3000)141 | 200-250 | N/A | Pro: Best in vivo recovery and survival Con: Short shelf life, bacterial growth, low in vitro quality |
| CSPs | Regular donor restrictions | Apheresis/phlebotomy | Additive solutions, leukoreduction | Liquid 1° to 6°C | 3 d, 14 d* | Therapeutic | Low143,144 | 200-250 | N/A | Pro: Extended shelf life, preserved in vitro quality Con: reduced in vivo recovery and survival, in vivo hemostatic function uncertain? |
| Cold-stored whole blood | Group O, low titer anti-A/B, possibly males only | Phlebotomy | Leukoreduction | Liquid 1°C to 6°C | 21 d (CPD), 35 d (CPDA-1) | Therapeutic (especially outside of hospital, trauma) | Low142 | 500 | N/A | Pro: Extended shelf life, includes all blood components Con: Possible ABO, Rh mismatch, lower recovery and survival, lower platelet dose, in vivo platelet function uncertain |
| CPPs | Regular donor restrictions | Apheresis/phlebotomy | Leukoreduction, resuspension in saline or plasma | Solid/frozen, −80°C | 3 y (≥5 y reported) | Therapeutic | Perceived to be very low16 | 20-50 | Thawing and resuspension† | Pro: Extended shelf life (years), almost normal in vivo survival, most in vivo performance data Con: logistics (freezer, thawing, preparation) |
| Freeze-dried (lyophilized) platelets‡ | Group O | Apheresis | Leukoreduction | Solid/powder, RT | 3 y | Therapeutic (especially outside of hospital, military) | Perceived to be very low (including pathogen reduction and culture)134 | 30-50 | Reconstitution in sterile water | Pro: Extended shelf life (years), logistics Con: reduced in vitro function, in vivo hemostatic function uncertain |
Variance for military and civilian blood centers.
Depending on preparation.
Thrombosomes.
Like platelet concentrates for component therapy, platelets in whole blood have been tested at 1°C to 6°C and at 20°C to 24°C with and without leukoreduction. Platelet function and procoagulant properties assessed in vitro are better preserved at 4°C than at 22°C for up to 14 to 21 days.82-89 Radiolabeling studies showed that recovery was higher in whole blood–derived platelets stored at 4°C than in 100% plasma-stored apheresis platelets at both day 10 (51% vs 31%) and day 15 (49% vs 22%) of cold storage.41,90 In vivo platelet survival was not different between the 2 groups.41,90 When comparing platelet concentrates to whole blood, one caveat is that whole blood contains only approximately one third to one fourth of an apheresis platelet dose, and therefore it has a much lower concentration of platelets. Leukoreduction of whole blood with a special platelet-sparing filter set reduces the platelet count by 20% to 30%91-93 and lowers the maximum amplitude in thromboelastography, hinting at a reduction in platelet function compared with unfiltered blood.92 Interestingly, storage in whole blood does not prevent the decrease in platelet counts during 4°C storage that many groups observed in plasma.41,60-64 In fact, some groups reported an even more pronounced loss of up to ∼60% of platelets within 10 days of storage in whole blood, likely because of the presence of plasma proteins in whole blood and the known ability of activated platelets to adhere to other cell types.82,88,90,94,95 Agitation seems dispensable for platelet count preservation in CSPs in plasma but is critical for CSPs in whole blood.90,96
Whole blood has other hemostatic properties beyond platelets and plasma. Duke97 reported that the prolonged bleeding time in patients with anemia or thrombocytopenia could be corrected by whole blood transfusion. RBCs have intrinsic prothrombotic properties but could also alter blood rheology and improve platelet margination.98 Indeed, preclinical models show that the number of platelets at the injury site and the duration of interaction between platelets and the injury site increase with a higher hematocrit.99 RBC-derived microparticles in stored RBCs promote coagulation.100,101 The combination of platelet-derived and RBC-derived microparticles in stored whole blood could be a potent procoagulant stimulant and more closely resemble physiological hemostasis.
Retrospective studies in trauma patients suggest no difference in hemolysis between ABO identical and minor ABO-incompatible transfusions of LTOWB. However, further investigations in clinical trials are warranted.102-104 A recent meta-analysis and systematic review of available randomized controlled trials comparing whole blood to component therapy concluded that there was no difference in safety and suggested a shorter duration of oxygen dependence, shorter duration of stay in the intensive care unit, and shorter hospital stay with whole blood transfusion.105 The certainty of the evidence was very low and the authors emphasized the need for data from larger, randomized controlled trials. Fortunately, several studies are in different stages of completion (PPOWER [NCT03477006], TOWAR [NCT04684719], and T-STOHRM106).
Cryopreserved (frozen) platelets
The first reports with CPPs date back to the 1950s,107 but 2 protocol modifications by Valeri108,109 significantly improved and simplified the preparation of CPPs. In brief, 6% dimethyl sulfoxide (DMSO) is added and platelets are concentrated to 10 mL and snap-frozen to prevent the formation of ice crystals. The unit is reconstituted by adding the thawed concentrate to 20 to 50 mL of saline or fresh-frozen plasma. Removing excess DMSO before freezing eliminates the need for post-thaw washing steps, which is time-consuming and can also cause platelet activation. Although it takes only 8 to 10 minutes to reconstitute CPPs, the need for thawed plasma is an important factor in some protocols. In some larger institutions, thawed plasma is always available. However, in smaller institutions, thawing plasma specifically for CPPs could add more time.110 The low volume of CPPs could be an advantage in patients at risk for transfusion-associated circulatory overload.
After cryopreservation, platelets change structure and shape, increase expression of phosphatidylserine and P-selectin on the outer membrane, and increase their procoagulant potential.111 When stimulated with agonists, they also show decreased aggregation.112 Similar to CSPs, CPPs differ in their immune characteristics when compared with RT-stored platelets.113
The Dutch military blood bank published their retrospective experience of transfusing 1143 units of CPPs to 349 patients in Afghanistan in 2006 and again a decade later.114,115 The product was reported to be safe and efficacious. The Czech Republic military found that transfusing CPPs or standard RT-stored platelets produced similar clinical outcomes in patients who experienced trauma or massive bleeding.116,117 Many other countries, including Australia, Belgium, Brazil, France, Turkey, Singapore, and the United States, have adopted similar programs predominantly for use in the military.
An important difference between CSPs and CPPs is their survival in circulation, which is only minimally reduced for CPPs, suggesting that some of the changes during the slow cooling of CSPs are bypassed in CPPs.112 One possibility is that the freezing process preserves glycans critical for platelet survival in vivo. A trauma-induced hemorrhage model showed no significant difference between CPPs and RT-stored platelets in preserving endothelial integrity.25 Khuri and colleagues118 conducted a randomized trial comparing standard RT-stored platelets and CPPs during cardiac surgery. Patients who received CPPs required fewer blood products and had reduced postoperative blood loss, but posttransfusion platelet increments and survival were lower than in the liquid-stored group. In a phase 1 randomized controlled clinical study from 2018, Slichter et al119 evaluated the safety and efficacy of transfusing CPPs in a dose-escalation manner to 28 patients with thrombocytopenia who had active bleeding (World Health Organization [WHO] grade 2-4). Minor adverse events were identified as being related to DMSO. Fifty-eight percent of the patients receiving CPPs had improved bleeding scores compared with 50% of the patients receiving standard RT platelets. Interestingly, bleeding also improved in 43% of patients with WHO grade 4 intracranial hemorrhage transfused with CPPs despite lower platelet count increments in the CPP group. However, similar to other randomized, controlled trials involving CPPs, the absolute number of patients was small, and the difference was not significant.
CPPs from different manufacturing sites and continents differ in specific in vitro characteristics.120 Two randomized controlled pilot trials from Australia and New Zealand were recently reported110,121; as pilot trials, their primary end points were protocol feasibility and safety. The authors reported no significant difference in bleeding but significantly more fresh-frozen plasma and platelet transfusion in the cryopreserved group in one of the trials.110 These findings will need to be confirmed in definitive studies. In vitro studies showed that CPPs generate more platelet microparticles with greater thrombin generation,122 thus raising concerns for increased thrombotic risk when transfusing CPPs. However, no such signals have been detected so far in any of the trials outlined above. Based on a literature review of 3000 CPP transfusions to 1334 patients, no thromboembolic events have yet been reported.123
An ongoing randomized controlled trial compared CPPs and liquid-stored platelets in controlling blood loss in patients who received cardiac surgery (CRYPTICS [NCT04709705]). The maximal duration of storage for CPPs has not been formally established, but some studies have shown stability after 5 years of storage at −80°C (Table 1).
Freeze-dried (lyophilized) platelets
Platelets require membrane stabilization with paraformaldehyde or trehalose to preserve platelet quality during lyophilization and rehydration.124 Trehalose is a disaccharide that enables organisms to endure periods of severe desiccation (there are other proprietary formulations125-127). The resulting hemostatic product can be stored as powder at RT for years and can be reconstituted in minutes with sterile water.128 This is a significant advantage compared with CPPs or CSPs, which require freezers or refrigerators, respectively. The paraformaldehyde-treated product has been evaluated in vitro and in vivo with promising results, but its current development is on hold.129 In contrast, the trehalose-treated product is undergoing further development (Thrombosomes, Cellphire, Rockville, MD). Thrombosomes are generated from a pool of 5 to 10 group O apheresis platelet units to avoid interference with recipient ABO antibodies. Ninety percent of the donor plasma is removed, and thermal treatment results in a 3-to-6-log reduction in viral load to prevent transfusion-transmitted infections.130 Thrombosomes have a shelf life of 3 years. Like CPPs, the low transfusion volume (30-50 mL) could be advantageous for volume-sensitive patients.
Thrombosomes morphologically resemble fresh platelets in electron microscopy studies, with a slight loss of cytoplasmic architecture but without functional mitochondria.131,132 Accordingly, aggregation and αIIbβ integrin activation are significantly lower than with fresh platelets.26,132 αIIbβ3 integrin levels are unchanged after lyophilization, but GPIbα levels are reduced by ∼50%.132,133 Lyophilized platelets adhere to porcine aorta tunica media under flow when perfused in platelet-depleted plasma, suggesting that GPVI and αIIβ1 levels are preserved.131 Adding thrombosomes to reconstituted whole blood reduces the number of platelets that adhered to collagen under flow, hinting at a competitive inhibitory mechanism.132 The vast majority of thrombosomes are positive for phosphatidylserine at baseline, which provides a procoagulant surface.26,132,133 Thrombosomes contain large amounts of non-platelet microparticles of unclear origin.132 Whether the remaining integrin-mediated function is enough to promote primary hemostasis or whether the procoagulant activity is the predominant mechanism of action is unknown. Surprisingly, according to a recent trial, transfusion of freeze-dried platelets did not increase thrombin generation in recipients.130
Approximately 40% of thrombosomes can be detected 2 hours after transfusion in rabbits with circulation times of up to 24 hours, but data from the literature are contradictory.130,133,134 Contrary to CSPs and CPPs, the circulation time of lyophilized platelets has never been systematically addressed in human radiolabeling studies. Functionally, thrombosomes are incorporated into growing thrombin in mice and promote endothelial function and vascular integrity in animal models and in vitro.26 Species-specific thrombosomes significantly increased the hematocrit 48 hours after injury in a swine bleeding model, possibly because of reduced postoperative bleeding, but no difference was observed for short-term bleeding.135
Animal studies suggest that thrombosomes have a favorable safety profile, contrary to previous lyophilization preparations. In a healthy human dose-escalation study, up to one tenth of the lowest efficacious dose was tested. Only non-severe adverse events were noted, including a platelet antibody without associated thrombocytopenia. All adverse events were resolved by day 21.136 In a recent phase 1 trial, allogeneic thrombosomes were transfused at 3 dose levels in 3 cohorts, each consisting of 8 patients with hematologic malignancies.130 The WHO bleeding score improved in 63% but remained unchanged in 37% of patients through day 6 after transfusion with thrombosomes. Exclusion criteria were active infections, graft-versus-host disease, and coagulopathy, among others. No safety concerns were reported in this patient population at the doses tested. Similar to the findings in an injured swine model,137 there was a trend for less bleeding, mainly after 6 days. The apparent effect of thrombosomes on long-term but not short-term bleeding suggests that this was either a thrombosome-independent effect or an indicator that hitherto unexplored mechanisms play a role. Thrombosomes may need time to recover function in vivo (similar to liquid-stored platelets).
Stem-cell–derived megakaryocytes or platelets
Innovative methods of procuring in vitro genetically engineered megakaryocytes and platelets have been proposed and developed in recent years. CD34+ hematopoietic progenitor cells can be sourced from peripheral blood, umbilical cord blood, bone marrow, and fetal liver cells. The downsides of this approach are limited proliferation potential and continued dependence on donor material. Conversely, embryonic stem cells and the more recent advent of induced pluripotent stem cells allow for almost unlimited proliferation potential. A risk for alloimmunization accompanies transfusion of donor-derived cellular blood products. Generating platelets from stem cells promises to yield universally compatible platelets. This approach could prevent platelet clearance in previously alloimmunized patients or avoid initial alloimmunization. Platelets from earlier developmental stages, including embryonic platelets generated ex vivo, are hyporeactive and show other differences compared with adult platelets, such as reduced P-selectin levels.137-139 Future studies will need to investigate whether these differences are beneficial or detrimental to transfusion outcomes. In a recent trial, adult platelets worsened outcomes in premature infants, highlighting that age-dependent platelet phenotypes could be clinically relevant for transfusion practice.27 Major bottlenecks that still remain are high costs and low in vitro yield of platelets per megakaryocyte. See Figueiredo et al140 for an overview of differentiation protocols for the in vitro generation of megakaryocytes and platelets.
Summary
We show in this review that the platelet field has made advancements in understanding the effect of different storage conditions on the respective post-storage platelet phenotype. Clinical data for the safety and efficacy of alternative platelet products are accumulating. In the future, transfusion services will likely not be limited to one product but will have multiple products at their disposal, depending on site-specific patient populations and logistical requirements. One limitation of this review is the main focus on blood donor–derived products. Numerous ongoing research programs are attempting to circumvent the need for blood donors by generating platelets or megakaryocytes from induced pluripotent stem cells or by testing platelet antigen-coated beads or artificial platelets. In addition, promising pharmacologic additions, such as tranexamic acid, are currently under investigation. We believe that stem cell–based approaches, such as stem cell–derived platelets ex vivo, are fascinating long-term avenues to pursue and that donor-derived products offer an immediate to mid-term perspective and may even coexist with bioreactor-derived products in the future. Data on thrombogenicity and immunogenicity of alternative platelet products are of great concern, and larger trials are necessary for a more thorough assessment of these important questions.
Acknowledgments
The authors acknowledge John R. Hess for providing invaluable feedback and thank Jeffrey Miles and S. Lawrence Bailey for proofreading the manuscript and Renetta Stevens and Tena Petersen for administrative support.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (1R01HL153072-01), the Department of Defense (W81XWH-12-1-0441, EDMS 5570), and by the American Society of Hematology Scholar Award.
Authorship
Contribution: V.J.K. and M.S. wrote the manuscript.
Conflict-of-interest disclosure: M.S. received research funding from Cerus and Terumo BCT. The remaining author declares no competing financial interests.
Correspondence: Moritz Stolla, Bloodworks Northwest Research Institute, 1551 Eastlake Ave, Suite 100, Seattle, WA 98102; e-mail: mstolla@bloodworksnw.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal