Key Points
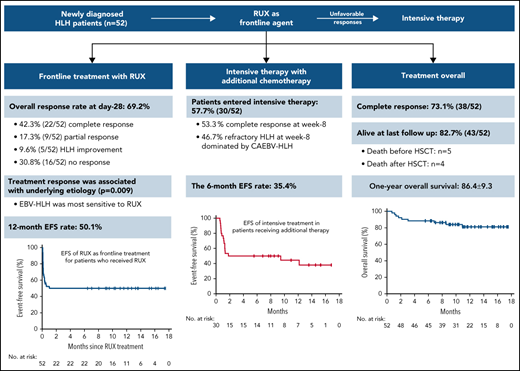
The ruxolitinib response–based stratified therapy achieved CR in 73.1% of HLH patients, with a 12-month survival of 86.4%.
Ruxolitinib, as a first-line agent, had a rapid efficacy for pediatric HLH and led to sustained CR in 42.3% of patients with good tolerance.
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a lethal disorder characterized by hyperinflammation. Recently, ruxolitinib (RUX), targeting key cytokines in HLH, has shown promise for HLH treatment. However, there is a lack of robust clinical trials evaluating its efficacy, especially its utility as a frontline therapy. In this study (www.chictr.org.cn, ChiCTR2000031702), we designed ruxolitinib as a first-line agent for pediatric HLH and stratified the treatment based on its early response. Fifty-two newly diagnosed patients were enrolled. The overall response rate (ORR) of ruxolitinib monotherapy (day 28) was 69.2% (36/52), with 42.3% (22/52) achieving sustained complete remission (CR). All responders achieved their first response to ruxolitinib within 3 days. The response to ruxolitinib was significantly associated with the underlying etiology at enrollment (P = .009). Epstein-Barr virus (EBV)-HLH patients were most sensitive to ruxolitinib, with an ORR of 87.5% (58.3% in CR). After ruxolitinib therapy, 57.7% (30/52) of the patients entered intensive therapy with additional chemotherapy. Among them, 53.3% (16/30) patients achieved CR, and 46.7% (14/30) patients dominated by chronic active EBV infection-associated HLH (CAEBV-HLH) developed refractory HLH by week 8. The median interval to additional treatment since the first ruxolitinib administration was 6 days (range, 3-25 days). Altogether, 73.1% (38/52) of the enrolled patients achieved CR after treatment overall. The 12-month overall survival (OS) for all patients was 86.4% (95% confidence interval [CI], 77.1% to 95.7%). Ruxolitinib had low toxicity and was well tolerated compared with intensive chemotherapy. Our study provides clinical evidence for ruxolitinib as a frontline agent for pediatric HLH. The efficacy was particularly exemplified with stratified regimens based on the early differential response to ruxolitinib. This study was registered in the Chinese Clinical Trials Registry Platform (http://www.chictr.org.cn/) as ChiCTR2000031702.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a lethal disorder characterized by pathologic immune activation and hyperinflammation. If left untreated, the uncontrolled inflammatory response can cause massive tissue injury and death.1 Etoposide-based HLH-1994 and HLH-2004 regimens remain widely accepted as the standard of treatment, which has substantially improved the survival of patients with this fatal condition. Nevertheless, quite a few patients are refractory to treatment or unable to tolerate intensive chemotherapy in current regimens.2,3 Furthermore, in many complicated scenarios, such as active fatal bleeding, patients are not candidates for intensive chemotherapy, which presents a dilemma of the requirement of immediate treatment and the avoidance of toxic chemicals. Along with a deeper understanding of the immunopathology of HLH, more specific therapies targeting key cytokines, such as anti-IFN–γ, interleukin-18 (IL-18) binding protein, and IL-1 and IL-6 inhibitors, have arisen as options for HLH treatment even though most of these agents are still being tested in clinical trials.4-7
As HLH is associated with the excessive production of numerous cytokines, the blockade of a broad array of disease-inducing cytokines may result in dramatic improvements in therapeutic efficacy. Ruxolitinib (RUX), a Janus kinase (JAK) 1 and 2 inhibitor, represents a promising therapeutic option for HLH, as RUX can inhibit signaling of both IFN-γ and other key proinflammatory cytokines involved in HLH via inhibition of the JAK1/2-STAT1 pathway. Data from HLH animal models have confirmed that this small molecule is a suitable agent for HLH treatment.8-10 The benefits of using RUX to control refractory or recent-onset HLH have also been reported in a series of HLH cases.11-15 Several clinical trials exploring RUX for HLH treatment are ongoing, and the preliminary data suggest that RUX is active and safe in their settings.16-19 According to a recent review, most of these studies have focused on the administration of RUX as salvage therapy in adult patients.20 There is still a lack of robust data on the utility of RUX monotherapy as frontline therapy, especially in children. Most importantly, we were eager to know if prioritizing patients for additional chemotherapy based on their response to RUX would achieve the most beneficial outcome.
In this study, we designed a single-arm, prospective study to explore the use of RUX monotherapy as a frontline therapy for pediatric HLH and stratified the patients for further treatment based on the early RUX response. Here, we reported the efficacy and safety of RUX response–based stratified treatment in a large cohort of 52 HLH children.
Methods
Study design and patients
This is a prospective, single-arm nonrandomized, phase 2 clinical trial (chictr.org.cn identifier: ChiCTR2000031702) performed at Beijing Children’s Hospital in China. This study followed the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Beijing Children’s Hospital. Informed consent was signed by the patients’ legal guardians.
Patients in this study were newly diagnosed HLH patients without prior chemotherapy for HLH before the screening. The detailed inclusion and exclusion criteria are shown in Table 1. The diagnostic criteria for underlying etiology, including systemic autoinflammatory disorder-associated HLH (SAIDs-HLH), Epstein-Barr virus-associated HLH (EBV-HLH), and chronic active EBV infection-associated HLH (CAEBV-HLH), are described in Table 2.
Eligibility criteria
Inclusion criteria
|
| Exclusion criteria Patients who had any one of the following were ineligible:
|
Inclusion criteria
|
| Exclusion criteria Patients who had any one of the following were ineligible:
|
Diagnostic criteria for primary diseases
SAIDs-HLH: meet all the following criteria:
|
CAEBV-HLH: meet all the following criteria:
|
EBV-HLH: meet all the following criteria:
|
SAIDs-HLH: meet all the following criteria:
|
CAEBV-HLH: meet all the following criteria:
|
EBV-HLH: meet all the following criteria:
|
Treatment procedures and response assessment
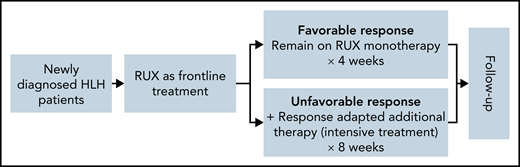
The treatment was stratified into 2 sequential phases: frontline treatment with RUX monotherapy (planned for 28 days) and individualized intensive treatment with RUX and additional chemotherapy (Figure 1). For the frontline treatment, all enrolled patients received a starting oral dose of RUX at 2.5 mg, 5 mg, or 10 mg every 12 hours depending on the body weight (≤10 kg, ≤20 kg, or >20 kg, respectively). Corticosteroid could be continued if the patient was receiving it before enrollment and was tapered gradually. The corticosteroid used in this protocol was methylprednisolone. Disease response evaluations were performed on days 3, 7, 14, and 28 after RUX therapy. Patients who had a favorable response remained on RUX monotherapy for a total of 4 weeks. For patients with unfavorable responses, additional chemotherapy for HLH was added (intensive treatment) for 8 weeks. Additional therapy was individualized based on the patient response and clinical assessments. Unfavorable responses were defined as follows: no response after 3 days of treatment; disease improvement but partial response was not achieved; or disease progression at any time during frontline treatment. Patients received the study treatment until treatment failure, intolerable toxicity, death, or withdrawal of consent occurred.
Treatment protocol stratified by the early responses of RUX. All enrolled patients received oral RUX as a frontline therapy, which was dosed depending on the body weight. Corticosteroid was permitted to continue if the patient was receiving it at screening and tapered gradually. Patients with a favorable response remained on RUX monotherapy for 4 weeks. Patients with an unfavorable response proceeded to intensive treatment with RUX and additional therapy. A favorable response was defined as achieving CR; an unfavorable response was defined as no response, disease improvement but not achieving PR, or disease progression at any time during frontline treatment.
Treatment protocol stratified by the early responses of RUX. All enrolled patients received oral RUX as a frontline therapy, which was dosed depending on the body weight. Corticosteroid was permitted to continue if the patient was receiving it at screening and tapered gradually. Patients with a favorable response remained on RUX monotherapy for 4 weeks. Patients with an unfavorable response proceeded to intensive treatment with RUX and additional therapy. A favorable response was defined as achieving CR; an unfavorable response was defined as no response, disease improvement but not achieving PR, or disease progression at any time during frontline treatment.
Treatment responses included complete response (CR), partial response (PR), HLH improvement, progressive disease, and relapse. The assessment criteria for treatment response are outlined in supplemental Table 1, which was mainly based on the criteria previously described in studies for pediatric HLH4 with modifications based on our experience.
Study endpoints
The primary endpoint was the overall response rate (ORR) at the end of frontline RUX monotherapy, including the proportion of patients achieving CR, PR, and HLH improvement. The key secondary endpoints were safety, the proportion of patients achieving CR after treatment overall, and the 12-month overall survival (OS) of the treatment protocol. Additional secondary endpoints for frontline RUX monotherapy included the time to response, the duration of response, the correlation of RUX responses with baseline characteristics, and the 12-month event-free survival (EFS). Additional secondary endpoints for intensive treatment included the proportion of patients achieving CR, the drug use of additional treatment, the number of patients proceeding to transplantation, the cause of death, and the 6-month EFS.
Events were defined as follows: no response, disease progression, relapse, or death for any reason. For frontline RUX monotherapy, EFS was estimated from the date of first-dose RUX administration to the date of any of the above events, whichever occurred first, or the date of the last follow-up. For intensive treatment, EFS was estimated from the date of additional treatment initiation to the date of any of the above events, whichever occurred first, or the date of the last follow-up.
Statistical analysis
The data were analyzed using SPSS Statistics software. Descriptive analyses are presented as the means and standard deviations for normally distributed variables and the medians (minimum and maximum) for variables with a skewed distribution. The number and percentage within each category are presented for the categorical variables. Continuous variables were compared with the t test or the Wilcoxon rank test, and categorical variables were compared with the χ-square test or Fisher exact test. Kaplan-Meier curves were used to estimate survival, and the differences in OS and EFS rates among different groups were compared by the log-rank test. All statistical tests were 2-tailed with a significance level of 0.05.
Results
Patient characteristics
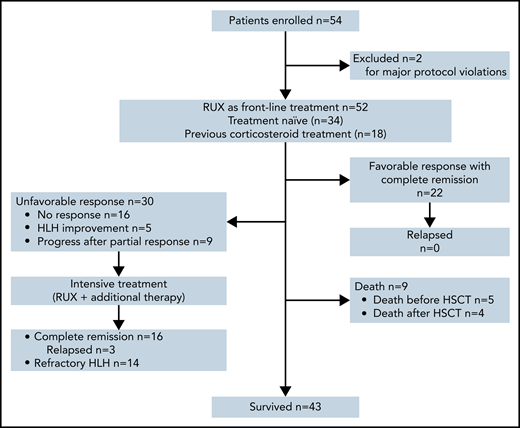
From 7 April 2020 to 1 October 2021, a total of 54 patients were enrolled in this study, and 2 (3.7%) patients were excluded for major protocol violations, data of which were not included in the analysis. A total of 52 patients entered the study of RUX frontline therapy, including 34 (65.4%) patients who had no prior treatment and 18 (34.6%) patients who were receiving corticosteroids at the time of screening. Among them, 30 (57.7%) patients entered intensive treatment with RUX in combination with additional chemotherapy because of unsatisfactory treatment responses (Figure 2). The median follow-up time was 11.2 months (range, 0.72-17.54). At the data cutoff, 43 patients (82.7%) were alive.
Summary of patient disposition. The data cutoff was 1 October 2021. A total of 54 patients were enrolled in this clinical trial, but 2 patients were excluded for major protocol violations, data of which were not included in the analysis of this study.
Summary of patient disposition. The data cutoff was 1 October 2021. A total of 54 patients were enrolled in this clinical trial, but 2 patients were excluded for major protocol violations, data of which were not included in the analysis of this study.
The baseline characteristics of the 52 patients are summarized in Table 3. The median age was 3.7 years (range, 0.1-14.4 years). The median time interval from onset to diagnosis was 19 days (range, 4-150 days). Typical HLH-associated clinical symptoms and laboratory features were present in all patients. The baseline characteristics of the patients were balanced between the prior corticosteroid-treated and untreated groups except for age, with a median age of 1.7 and 4.45, respectively (P = .008). Ten patients (19.2%) had CNS involvement at enrollment, mainly presenting as drowsiness and abnormal cerebrospinal fluid, but no obvious brain magnetic resonance imaging (MRI) abnormalities or neurological dysfunctions were observed. The underlying etiologies included 24 cases of EBV-HLH, 10 cases of CAEBV-HLH, 11 cases of SAIDs-HLH, and 7 cases of unknown etiology. Whole-exome sequencing was performed for all enrolled patients and their parents, and only 4 patients with EBV-HLH were found to have pathogenic gene mutations known to be associated with primary HLH after enrollment and treatment initiation (supplemental Table 2).
Baseline characteristics of the study population
| Patient subgroup . | Total (n = 52) . | Treatment naïve (n = 34) . | Previous corticosteroid treatment (n = 18) . | P value . |
|---|---|---|---|---|
| General | ||||
| Gender (male/female), n | 30/22 | 22/12 | 8/10 | .239 |
| Median age (range), y | 3.7 (0.1-14.4) | 4.45 (0.7-14.2) | 1.7 (0.1-14.4) | .008 |
| Duration before diagnosis, d | 19 (4-150) | 17.5 (4-100) | 23.5 (5-150) | .471 |
| Clinical parameters, % | ||||
| Fever (>38.5°C) | 100 | 100 | 100 | |
| Splenomegaly | 76.9 | 79.4 | 72.2 | .731 |
| Hepatomegaly | 73.1 | 76.5 | 66.7 | .519 |
| Lymphadenopathy | 80.8 | 82.4 | 77.8 | .723 |
| Jaundice | 19.2 | 20.6 | 16.7 | .521 |
| Rash | 40.4 | 35.5 | 50.0 | .378 |
| CNS involvement | 19.2 | 17.6 | 22.2 | .732 |
| Laboratory findings | ||||
| White blood cells, ×109/L | 2.84 (0.58-46.29) | 2.13 (0.58-14.14) | 7.64 (0.58-46.29) | .077 |
| Neutrophils, ×109/L | 0.87 (0.03-10.85) | 0.79 (0.03-8.83) | 1.08 (0.17-10.85) | .063 |
| Platelets, ×109/L | 74.5 (10-506) | 81.5 (10-506) | 56.5 (11-383) | .532 |
| Hemoglobin, g/L | 87.5 (55-129) | 89.5 (58-128) | 84 (55-129) | .222 |
| Fibrinogen, g/L | 1.6 (0.46-3.15) | 1.39 (0.47-3.08) | 1.72 (0.46-3.15) | .510 |
| Triglycerides, mmol/L | 2.86 (0.54-10.48) | 2.94 (0.76-10.48) | 2.67 (0.54-5.97) | .801 |
| AST, U/L | 208 (16-6790) | 223 (22-2037) | 187 (16-6790) | .373 |
| ALT, U/L | 156 (9.6-1918) | 171 (9.6-1044) | 143 (11-1918) | .309 |
| IFN-γ, increase (fold) | 38.5 (1.6-877.6) | 33.6 (1.6-877.6) | 42.6 (12.6-387.8) | .443 |
| Soluble CD25, increase (fold) | 6.2 (2.1-42.7) | 6.2 (2.1-41.8) | 5.6 (2.6-42.7) | .957 |
| Ferritin, increase (fold) | 8.0 (0.6-68.3) | 6.7 (0.6-58.3) | 8.3 (1.7-68.3) | .498 |
| Low NK cell activity, % | 75.0 | 76.5 | 72.2 | .747 |
| Hemophagocytosis, % | 76.9 | 82.4 | 66.7 | .300 |
| Patient subgroup . | Total (n = 52) . | Treatment naïve (n = 34) . | Previous corticosteroid treatment (n = 18) . | P value . |
|---|---|---|---|---|
| General | ||||
| Gender (male/female), n | 30/22 | 22/12 | 8/10 | .239 |
| Median age (range), y | 3.7 (0.1-14.4) | 4.45 (0.7-14.2) | 1.7 (0.1-14.4) | .008 |
| Duration before diagnosis, d | 19 (4-150) | 17.5 (4-100) | 23.5 (5-150) | .471 |
| Clinical parameters, % | ||||
| Fever (>38.5°C) | 100 | 100 | 100 | |
| Splenomegaly | 76.9 | 79.4 | 72.2 | .731 |
| Hepatomegaly | 73.1 | 76.5 | 66.7 | .519 |
| Lymphadenopathy | 80.8 | 82.4 | 77.8 | .723 |
| Jaundice | 19.2 | 20.6 | 16.7 | .521 |
| Rash | 40.4 | 35.5 | 50.0 | .378 |
| CNS involvement | 19.2 | 17.6 | 22.2 | .732 |
| Laboratory findings | ||||
| White blood cells, ×109/L | 2.84 (0.58-46.29) | 2.13 (0.58-14.14) | 7.64 (0.58-46.29) | .077 |
| Neutrophils, ×109/L | 0.87 (0.03-10.85) | 0.79 (0.03-8.83) | 1.08 (0.17-10.85) | .063 |
| Platelets, ×109/L | 74.5 (10-506) | 81.5 (10-506) | 56.5 (11-383) | .532 |
| Hemoglobin, g/L | 87.5 (55-129) | 89.5 (58-128) | 84 (55-129) | .222 |
| Fibrinogen, g/L | 1.6 (0.46-3.15) | 1.39 (0.47-3.08) | 1.72 (0.46-3.15) | .510 |
| Triglycerides, mmol/L | 2.86 (0.54-10.48) | 2.94 (0.76-10.48) | 2.67 (0.54-5.97) | .801 |
| AST, U/L | 208 (16-6790) | 223 (22-2037) | 187 (16-6790) | .373 |
| ALT, U/L | 156 (9.6-1918) | 171 (9.6-1044) | 143 (11-1918) | .309 |
| IFN-γ, increase (fold) | 38.5 (1.6-877.6) | 33.6 (1.6-877.6) | 42.6 (12.6-387.8) | .443 |
| Soluble CD25, increase (fold) | 6.2 (2.1-42.7) | 6.2 (2.1-41.8) | 5.6 (2.6-42.7) | .957 |
| Ferritin, increase (fold) | 8.0 (0.6-68.3) | 6.7 (0.6-58.3) | 8.3 (1.7-68.3) | .498 |
| Low NK cell activity, % | 75.0 | 76.5 | 72.2 | .747 |
| Hemophagocytosis, % | 76.9 | 82.4 | 66.7 | .300 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; IFN-γ, interferon-γ; NK, natural killer.
Normal reference range by the clinical laboratory: AST ≤ 40 U/L; ALT ≤ 40 U/L; IFN-γ ≤ 8 pg/mL; ferritin ≤ 500 μg/L; soluble CD25 ≤ 6400 pg/mL; natural killer cell activity ≥15.11%. The baseline values of soluble CD25 and ferritin were described as “increase (fold),” which was calculated based on the upper limits of normal (6400 pg/mL and 500 μg/L, respectively).
Efficacy
Response to frontline RUX monotherapy
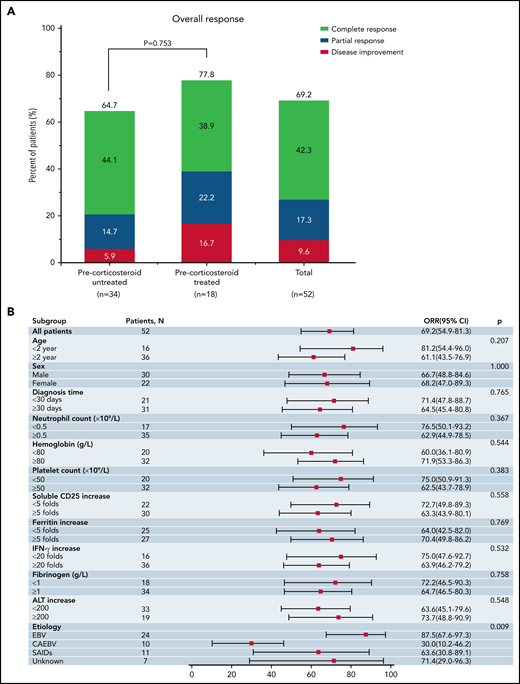
For the primary endpoints, the ORR at the end of RUX frontline therapy (day 28) was 69.2% (36/52 patients; 95% CI, 54.9% to 81.3%), with 42.3% (22/52 patients) achieving CR, 17.3% (9/52 patients) achieving PR, and 9.6% (5/52 patients) showing HLH improvement (Figure 3A; Table 4). The median time to CR was 21 days (range, 14-28 days). For subgroup analysis, the ORR exhibited no significant difference between the prior corticosteroid-treated and untreated groups (77.8% [95% CI, 52.4% to 93.6%] vs 64.7% [95% CI, 46.5% to 80.3%]; P = .753). Furthermore, there was also no significant difference between the 2 groups in terms of the proportion of patients who had a favorable response to RUX (achieving CR) (38.9% [7/18 patients] vs 44.1% [15/34 patients]; P = .775). Notably, 3 out of the 4 primary HLH patients achieved CR after RUX monotherapy.
Response outcomes of RUX frontline therapy. (A) ORR at the end of RUX frontline therapy (day 28). Two-sided P values were calculated by Wilcoxon rank-sum test. (B) Subgroup analysis of the association between day 28 ORR and clinical characteristics of the patients at baseline. Two-sided P values were calculated by χ-square test.
Response outcomes of RUX frontline therapy. (A) ORR at the end of RUX frontline therapy (day 28). Two-sided P values were calculated by Wilcoxon rank-sum test. (B) Subgroup analysis of the association between day 28 ORR and clinical characteristics of the patients at baseline. Two-sided P values were calculated by χ-square test.
Clinical outcome
| Outcome . | Prior corticosteroid untreated . | Prior corticosteroid treated . | Total . |
|---|---|---|---|
| RUX as frontline treatment, n | 34 | 18 | 52 |
| Favorable response with CR at day 28, n (%) | 15 (44.1) | 7 (38.9) | 22 (42.3) |
| Time to achieve CR, d | 21 (21-28) | 21 (14-28) | 21 (14-28) |
| Duration of CR response, mo (range) | 13.3 (9.0-17.5) | 9.5 (6.4-16.4) | 11.9 (6.4-17.5) |
| Unfavorable response requiring additional HLH treatments before day 28, n (%) | 19 (55.9) | 11 (61.1) | 30 (57.7) |
| No response, n (%) | 12 (35.3) | 4 (22.2) | 16 (28.9) |
| Progressive disease after PR,* n (%) | 5 (14.7) | 4 (22.2) | 9 (13.5) |
| Disease improvement, n (%) | 2 (5.9) | 3 (16.7) | 5 (15.4) |
| Additional treatment start day since first RUX administration (range) | 5 (3-12) | 7.5 (3-25) | 6 (3-25) |
| Death, n (%) | 0 | 0 | 0 |
| RUX + additional chemotherapy, n | 19 | 11 | 30 |
| Achieve CR at week 8, n (%) | 10 (52.6) | 6 (54.5) | 16 (53.3) |
| Refractory HLH, n (%) | 9 (47.4) | 5 (45.5) | 14 (46.7) |
| Salvage HSCT, n (% of refractory HLH) | 5 | 3 | 8 (57.1) |
| Alive, n (% of HSCT) | 3 | 2 | 5 (62.5) |
| Without HSCT, n (% of refractory HLH) | 3 | 3 | 6 (42.9) |
| Alive, n (% of without HSCT) | 0 | 1 | 1 (16.7) |
| Overall treatment, n | 34 | 18 | 52 |
| Achieve CR, n (%) | 25 (73.5) | 13 (72.2) | 38 (73.1) |
| Relapse, n (% of CR) | 2 | 1 | 3 (8.1) |
| Alive at last follow, n (%) | 29 (85.3) | 14 (77.8) | 43 (82.7) |
| Death before HSCT due to active HLH, n (%) | 3 (8.8) | 2 (11.1) | 5 (9.6) |
| Death after HSCT, n (%) | 2 (5.9) | 2 (11.1) | 4 (7.7) |
| 12-mo cumulative survival (±95% CI) | — | — | 86.4 ± 9.3 |
| Outcome . | Prior corticosteroid untreated . | Prior corticosteroid treated . | Total . |
|---|---|---|---|
| RUX as frontline treatment, n | 34 | 18 | 52 |
| Favorable response with CR at day 28, n (%) | 15 (44.1) | 7 (38.9) | 22 (42.3) |
| Time to achieve CR, d | 21 (21-28) | 21 (14-28) | 21 (14-28) |
| Duration of CR response, mo (range) | 13.3 (9.0-17.5) | 9.5 (6.4-16.4) | 11.9 (6.4-17.5) |
| Unfavorable response requiring additional HLH treatments before day 28, n (%) | 19 (55.9) | 11 (61.1) | 30 (57.7) |
| No response, n (%) | 12 (35.3) | 4 (22.2) | 16 (28.9) |
| Progressive disease after PR,* n (%) | 5 (14.7) | 4 (22.2) | 9 (13.5) |
| Disease improvement, n (%) | 2 (5.9) | 3 (16.7) | 5 (15.4) |
| Additional treatment start day since first RUX administration (range) | 5 (3-12) | 7.5 (3-25) | 6 (3-25) |
| Death, n (%) | 0 | 0 | 0 |
| RUX + additional chemotherapy, n | 19 | 11 | 30 |
| Achieve CR at week 8, n (%) | 10 (52.6) | 6 (54.5) | 16 (53.3) |
| Refractory HLH, n (%) | 9 (47.4) | 5 (45.5) | 14 (46.7) |
| Salvage HSCT, n (% of refractory HLH) | 5 | 3 | 8 (57.1) |
| Alive, n (% of HSCT) | 3 | 2 | 5 (62.5) |
| Without HSCT, n (% of refractory HLH) | 3 | 3 | 6 (42.9) |
| Alive, n (% of without HSCT) | 0 | 1 | 1 (16.7) |
| Overall treatment, n | 34 | 18 | 52 |
| Achieve CR, n (%) | 25 (73.5) | 13 (72.2) | 38 (73.1) |
| Relapse, n (% of CR) | 2 | 1 | 3 (8.1) |
| Alive at last follow, n (%) | 29 (85.3) | 14 (77.8) | 43 (82.7) |
| Death before HSCT due to active HLH, n (%) | 3 (8.8) | 2 (11.1) | 5 (9.6) |
| Death after HSCT, n (%) | 2 (5.9) | 2 (11.1) | 4 (7.7) |
| 12-mo cumulative survival (±95% CI) | — | — | 86.4 ± 9.3 |
HSCT, hematopoietic stem cell transplantation.
All patients achieving PR with RUX frontline therapy developed a progressive disease before day 28.
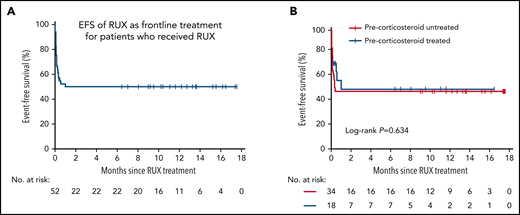
The median time to first response of all responders was 2 days (range, 1-3 days). Among the responders, all patients who achieved CR stopped RUX at day 28 and maintained CR throughout the entire follow-up period. All patients achieving PR (n = 9) eventually had an unfavorable response to RUX and developed progressive disease before day 28. Five patients received additional chemotherapy before the end of the frontline study due to the severity of their condition despite clinical improvement. The 12-month EFS rate for frontline RUX monotherapy was 50.1% (95% CI, 35.9% to 64.1%). There was no significant difference in the 12-month EFS between the prior corticosteroid-treated and untreated groups (50.6% [95% CI, 25.5% to 75.7%] vs 48.5% [95% CI, 31.3% to 65.7%]; P = .634) (Figure 4).
EFS of frontline treatment with RUX. (A) Kaplan-Meier estimated EFS in all patients who received RUX. (B) EFS in patients by treatment status at enrollment (precorticosteroid treated vs precorticosteroid untreated). No significant difference was detected between the 2 groups by the log-rank test (P = .634). For nonresponders, EFS was defined as the date of enrollment plus 1 day.
EFS of frontline treatment with RUX. (A) Kaplan-Meier estimated EFS in all patients who received RUX. (B) EFS in patients by treatment status at enrollment (precorticosteroid treated vs precorticosteroid untreated). No significant difference was detected between the 2 groups by the log-rank test (P = .634). For nonresponders, EFS was defined as the date of enrollment plus 1 day.
A dramatic resolution in HLH parameters was observed in CR patients compared with nonresponders (supplemental Figure 1). In particular, the levels of soluble CD25 (sCD25), serum ferritin, and interferon-γ (IFN-γ) showed rapid improvement in kinetics, decreasing to the normal range within 1 to 2 weeks. For patients who achieved PR and later relapsed, rapid refevers (not shown) and abnormal increases in IFN-γ, sCD25, and serum ferritin levels were detected. These results indicate that these parameters may be sensitive markers for evaluating the response to RUX in the early phase. In contrast, cytopenia, coagulopathy, and splenomegaly showed slower improvement kinetics with granulocyte colony-stimulating factor injection or blood transfusion support, especially hemoglobin, fibrinogen, and splenomegaly, which reached normal levels for 4 weeks. Although RUX was reported to cause high triglyceride levels, the levels decreased to normal within 2 weeks in most individuals in this study.
For exploratory analysis, the baseline characteristics demonstrated that the ORR on day 28 was associated with disease etiology at enrollment (P = .009) (Figure 3B). No significant association with the other factors evaluated was observed, including the time to diagnosis, potential CNS involvement, and the levels of sCD25, ferritin, and IFN-γ (Figure 3B). In detail, patients with EBV-HLH were most sensitive to RUX, with an ORR of 87.5%, while patients with CAEBV-HLH had the worst response, with an ORR of 30%. SAIDs-HLH patients and those with unknown etiology had ORRs of 63.6% and 71.4%, respectively. Furthermore, to identify the clinical characteristics of patients who had a favorable response to RUX, the baseline HLH features were compared by RUX response status. As shown in supplemental Figure 2, except for the interval from onset to diagnosis, there were no significant differences in the baseline characteristics between patients with and those without favorable responses to RUX (achieving CR). The median interval from onset to diagnosis in favorable and unfavorable responders was 14 days (range, 2-48 days) and 28.5 days (range, 7-150 days), respectively, indicating a potentially better RUX response with earlier intervention (P = .003). Interestingly, when grouped by underlying etiology, patients with favorable responses to RUX were dominated by EBV-HLH cases (63.6% [14/22]). The percentage of EBV-HLH patients with a favorable response (58.3%) was significantly higher than that of both SAIDs-HLH (27.3%) and CAEBV-HLH (0%) patients (P = .003) (supplemental Figure 3). Notably, 57.1% (8/14) of the EBV-HLH patients with favorable response presented with fulminant primary EBV infection, indicated by EBV-specific serology with positive EBV viral capsid antigen (VCA) immunoglobulin M (IgM) or VCA IgG of low affinity in the absence of EBV nuclear antigen IgG.
Response to intensive therapy
After frontline RUX monotherapy, 22 (42.3%) of the 52 patients responded well to RUX without additional chemotherapy. However, there were still 30 (57.7%) of 52 patients who proceeded to intensive treatment because of unfavorable responses to RUX, including 16 (30.8%) patients with no response, 5 (9.6%) patients with marginal HLH improvement, and 9 (17.3%) patients with progressive disease after PR (Table 4). The median interval from the first RUX administration to additional treatment was 6 days (range, 3-25 days). Additional HLH treatments included methylprednisolone and etoposide (used in the majority of patients), cyclosporine A (used only in SAIDs patients), and liposomal doxorubicin and pegaspargase (used in refractory patients). The dose for the intensive treatment and the outcomes for individual patients are outlined in supplemental Table 3.
By week 8, 53.3% (16/30) of these patients responded well to subsequent intensive treatment and achieved CR, while 46.7% (14/30) developed refractory HLH with unfavorable responses to intensive treatment (Table 4). Among the patients achieving CR, 1 patient (SAIDs-HLH) received only additional methylprednisolone, 5 patients (SAIDs-HLH) received methylprednisolone and cyclosporine A (1 of them subjected to etoposide later), and 10 patients received methylprednisolone and etoposide. The initial dose of methylprednisolone was 2 mg/kg per day, and that of etoposide (if needed) was 100 mg/m2 per dose. The median number of etoposide doses administered to those patients was 4.5 (range, 0-11 doses). For refractory patients, the median interval from the time of the first intensive treatment to the addition of liposomal doxorubicin and PEG-asparaginase was 3.5 weeks (range, 2.0-5.0 weeks). Among all refractory HLH patients, 57.1% (8/14) of patients proceeded to salvage hematopoietic stem cell transplantation (HSCT) (5 survived [62.5%]), and 42.9% (6/14) of patients did not undergo HSCT (1 survived [16.7%]) (Table 4). Reasons for not undergoing HSCT were financial difficulty (n = 3), serious infection (n = 2), and tocilizumab treatment (n = 1).
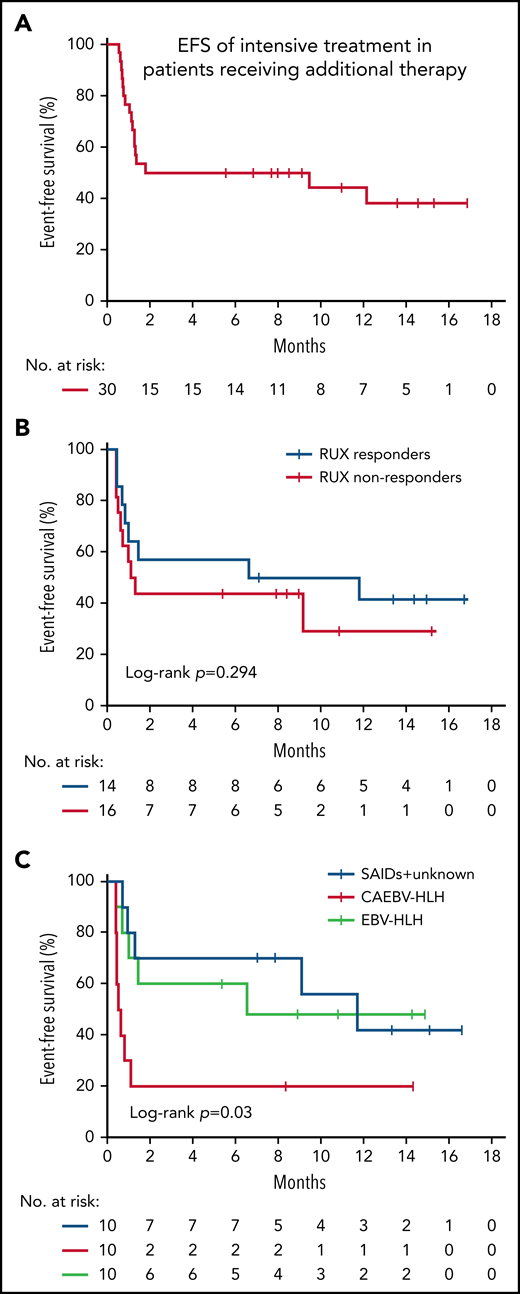
The 6-month EFS rate of intensive treatment in those patients was 35.4% (95% CI, 16.2% to 54.6%) (Figure 5A). To explore whether the status of RUX response before entering intensive treatment was related to the outcome of additional chemotherapy, we analyzed the EFS between RUX responders and nonresponders up to day 28. No statistical differences in the 6-month EFS rate were observed between the 2 groups, although the EFS was numerically higher in RUX responders than in RUX nonresponders (57.1% ± 13.2% vs 43.8% ± 12.4%; P = .294) (Figure 5B). However, there were significant differences in the 6-month EFS among patients with different underlying diseases (P = .03) (Figure 5C), which were greatly better in the EBV-HLH and SAIDs-HLH patients than in the CAEBV-HLH patients. These results suggest that underlying etiology could serve as a prognostic factor for the intensive treatment in unfavorable responders to RUX.
EFS of subsequent intensive treatment. (A) EFS of patients who had an unfavorable response to RUX and received intensive treatment. EFS was estimated from the date of additional treatment initiation to the first occurrence of disease progression, relapse, or death, or the date of the last follow-up. (B) EFS of intensive treatment by RUX response status (responders vs nonresponders) before entering intensive treatment. (C) EFS of intensive treatment by underlying etiology.
EFS of subsequent intensive treatment. (A) EFS of patients who had an unfavorable response to RUX and received intensive treatment. EFS was estimated from the date of additional treatment initiation to the first occurrence of disease progression, relapse, or death, or the date of the last follow-up. (B) EFS of intensive treatment by RUX response status (responders vs nonresponders) before entering intensive treatment. (C) EFS of intensive treatment by underlying etiology.
Overall outcome and survival
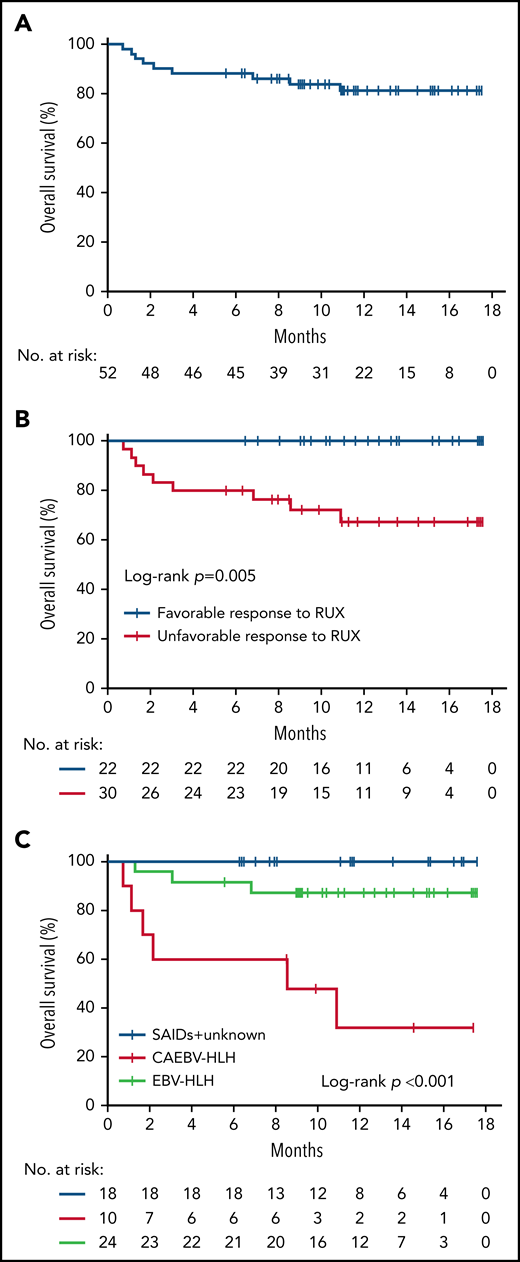
Altogether, 38 (73.1%) of the 52 enrolled patients achieved CR at the end of treatment, and 43 (82.7%) of the 52 patients were alive at the last follow-up (Table 4). Five patients died before HSCT due to multiple organ failure secondary to persistent HLH activation (n = 3) or severe infection (n = 2). Four patients died after HSCT due to severe graft-versus-host disease (n = 1), or uncontrollable relapsed HLH after HSCT (n = 3) (supplemental Table 3). The 12-month OS of all patients was 86.4% (95% CI, 77.1% to 95.7%) (Figure 6A). For subgroup analysis, the 12-month OS of patients who had a favorable response to RUX at day 28 was higher than that of unfavorable responders (100% vs 76.4% [95% CI, 61.1% to 90.9%]; P = .005) (Figure 6B), indicating that RUX response status is significantly associated with OS. In addition, the underlying etiology was also significantly associated with OS (P < .001), with 32.0% (95% CI, 15.5% to 48.5%) in the CAEBV-HLH group, 87.3% (95% CI, 75.8% to 98.8%) in the EBV-HLH group, and 100% in SAIDs and other types group, respectively (Figure 6C).
Kaplan-Meier analysis of OS. (A) The OS of treatment overall. (B) Comparison of the OS in patients by RUX monotherapy response status (favorable responders vs unfavorable responders). (C) Comparison of the OS in patients by underlying etiology.
Kaplan-Meier analysis of OS. (A) The OS of treatment overall. (B) Comparison of the OS in patients by RUX monotherapy response status (favorable responders vs unfavorable responders). (C) Comparison of the OS in patients by underlying etiology.
The overall outcome of this RUX-based treatment was compared with that of the historical HLH-1994 study,2 both of which enrolled patients who did not receive any prior chemotherapy (supplemental Table 4). No significant difference in the CR rate at the end of treatment (8 weeks) was observed between the 2 studies, although patients treated with the RUX-based protocol had a numerically higher CR rate (38/52 [73.1%] vs 122/207 [59.0%]; P = .061). The alive rates at both 8 weeks and 1 year in the RUX-based treatment were comparable to those reported in the HLH-1994 study (90.4% [47/52] vs 86.0% [214/249]; P = .391; and 82.7% [43/52] vs 74.3% [185/249]; P = .199, respectively). Notably, there were still some imbalances in enrollment between the 2 studies. First, the percentage of primary HLH patients enrolled in this study was obviously lower than that in the HLH-1994 study (7.7% vs 24.0%; P = .009). In addition, patients with severe CNS involvement were excluded from this study in contrast to HLH-1994.
Safety
For safety endpoints, we analyzed the adverse events (AEs) in patients who received RUX only (n = 22) and those who received RUX and additional chemotherapy (n = 30), respectively. All possible AEs are summarized in Table 5. Since HLH is characterized by systemic tissue injury, some of the AEs may be attributable to HLH activation or coexisting conditions of disease activation and drug side effects. In the RUX monotherapy group, all patients received RUX for up to 28 days. Treatment was well-tolerated, and most AEs were grade 1/2 in this group. The most frequent AEs up to day 28 in these patients were constipation (45.5%), pancreatic damage (36.4%), anemia (31.8%), and thrombocytopenia (22.7%). Most patients with pancreatic damage only exhibited abnormal increases of serum amylase and lipase, without pancreatitis manifestations or imaging abnormalities. Only 1 patient had grade 3 pancreatic damage, presenting as acute pancreatitis with abdominal pain. MRI of the pancreas showed obvious pancreatic swelling and hyperintense lesions. This patient was treated with somatostatin and enteral nutrition without RUX discontinuation. Her pancreatitis was not resolved after 2 weeks of treatment. However, after RUX cessation on day 28 as planned with HLH CR, the pancreatic damage was improved rapidly, and the patient was discharged from the hospital soon after. No other patients in this group had grade 3 or higher AEs of organ toxicity or secondary infection. In contrast, AEs were reported in most patients in the additional treatment group, which are usually observed with conventional therapy. The most common AEs were myelosuppression (73.3%), secondary infection (56.7%), sweating (53.3%), and liver damage (46.7%). Furthermore, more patients developed grade 3 or higher AEs up to week 8, including 11 (36.7%) patients with myelosuppression, 7 (23.3%) patients with severe infection, 6 (20.0%) patients with pancreatic damage, 5 (16.7%) patients with liver damage, and 3 (10.0%) patients with gastrointestinal hemorrhage.
Possible adverse events*
| Event . | Ruxolitinib (n = 22), n (%) . | Ruxolitinib + additional therapy (n = 30), n (%) . | ||
|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Hematologic AEs | ||||
| Anemia | 7 (31.8) | 4 (18.2) | 13 (43.3) | 6 (20.0) |
| Thrombocytopenia | 5 (22.7) | 2 (9.1) | 15 (50.0) | 8 (26.7) |
| Neutrocytopenia | 4 (18.2) | 0 | 22 (73.3) | 10 (33.3) |
| Myelosuppression | 0 | 0 | 22 (73.3) | 11 (36.7) |
| Nonhematologic abnormalities | ||||
| Constipation | 10 (45.5) | 0 | 9 (30.0) | 0 |
| Pancreatic damage | 8 (36.4) | 1 (4.5) | 10 (33.3) | 6 (20.0) |
| Rash | 4 (18.2) | 0 | 7 (23.3) | 0 |
| Diarrhea | 3 (13.6) | 0 | 9 (30.0) | 0 |
| Liver damage | 3 (13.6) | 0 | 14 (46.7) | 5 (16.7) |
| Sweating | 2 (9.1) | 0 | 16 (53.3) | 0 |
| Gastritis | 1 (4.5) | 0 | 0 | 0 |
| Secondary infection | 0 | 0 | 17 (56.7) | 7 (23.3) |
| Heart damage | 0 | 0 | 6 (20.0) | 0 |
| Kidney damage | 0 | 0 | 4 (13.3) | 0 |
| Gastrointestinal hemorrhage | 0 | 0 | 5 (16.7) | 3 (10.0) |
| Event . | Ruxolitinib (n = 22), n (%) . | Ruxolitinib + additional therapy (n = 30), n (%) . | ||
|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Hematologic AEs | ||||
| Anemia | 7 (31.8) | 4 (18.2) | 13 (43.3) | 6 (20.0) |
| Thrombocytopenia | 5 (22.7) | 2 (9.1) | 15 (50.0) | 8 (26.7) |
| Neutrocytopenia | 4 (18.2) | 0 | 22 (73.3) | 10 (33.3) |
| Myelosuppression | 0 | 0 | 22 (73.3) | 11 (36.7) |
| Nonhematologic abnormalities | ||||
| Constipation | 10 (45.5) | 0 | 9 (30.0) | 0 |
| Pancreatic damage | 8 (36.4) | 1 (4.5) | 10 (33.3) | 6 (20.0) |
| Rash | 4 (18.2) | 0 | 7 (23.3) | 0 |
| Diarrhea | 3 (13.6) | 0 | 9 (30.0) | 0 |
| Liver damage | 3 (13.6) | 0 | 14 (46.7) | 5 (16.7) |
| Sweating | 2 (9.1) | 0 | 16 (53.3) | 0 |
| Gastritis | 1 (4.5) | 0 | 0 | 0 |
| Secondary infection | 0 | 0 | 17 (56.7) | 7 (23.3) |
| Heart damage | 0 | 0 | 6 (20.0) | 0 |
| Kidney damage | 0 | 0 | 4 (13.3) | 0 |
| Gastrointestinal hemorrhage | 0 | 0 | 5 (16.7) | 3 (10.0) |
Some patients had more than 1 AE.
Since HLH is characterized by systemic tissue injury, including cytopenia, liver damage, and other organ involvement, some of the AEs may be attributable to HLH activation or coexisting conditions of disease and drug.
Intention-to-treat analyses
All enrolled patients (n = 54) were included in the intention-to-treat analyses. The primary and main secondary outcomes were similar to those of the above analyses, excluding the 2 patients with protocol violations (supplemental Table 5; supplemental Data).
Discussion
In this study, we designed RUX as a first-line agent for HLH in children and stratified the subsequent treatment based on the responses to RUX. This is the first and largest cohort study demonstrating meaningful and clinical benefits of such a RUX response–based stratified treatment approach for HLH. In this study, the overall response to RUX monotherapy on day 28 was 69.2%. Strikingly, all responding patients achieved their first response within 3 days, indicating that RUX had a quick effect on HLH, thereby allowing for rapid identification of nonresponders. This feature makes it possible for RUX to serve as an excellent indicator for treatment stratification based on its early responses. After treatment overall, a total of 73.1% (38/52) of patients achieved CR, of whom 42.3% (22/52) responded well to RUX monotherapy and maintained CR. The results demonstrate that there is a considerable subgroup of patients who will respond well to RUX as a frontline therapy and benefit from avoiding unnecessary chemotherapy. Notably, all patients who achieved PR with RUX monotherapy eventually developed progressive disease. The low dose of RUX used in this study and individual differences in the pharmacokinetic response to RUX may be possible reasons for disease reactivation. Moreover, although RUX lessened inflammation rapidly, elimination of the underlying triggers while suppressing hyperinflammation is also necessary for the treatment of secondary HLH. Thus, failure to control the underlying disease may also contribute to disease progression.
Due to the considerable heterogeneity in HLH, it is important to identify the specific HLH settings sensitive to RUX to guide better treatment. Our results showed that the efficacy of RUX did not appear to be associated with disease severity, as there was no significant difference in baseline characteristics between the favorable and unfavorable responders. In support of this notion, additional studies have demonstrated that RUX, serving as salvage therapy, can induce remission in some refractory HLH patients.11-13,22,23 In contrast, in this study, the response to RUX treatment was significantly associated with the underlying etiology at enrollment. Compared with other subtypes, EBV-HLH tends to be more sensitive to RUX, showing a higher percentage of a favorable response. Notably, the majority of these EBV-HLH patients had fulminant primary EBV infection. Primary EBV infection often manifests as infectious mononucleosis, with the majority of cases running a self-limiting course with or without supportive therapy.24 However, sustained primary EBV infection can also trigger immediately fatal or life-threatening HLH, especially in patients with unknown congenital or acquired immunodeficiencies,25-27 thus requiring prompt HLH treatment. As primary EBV infection is a typical feature of the pediatric population compared with adults, our results suggest that RUX may have different efficacies in children and adults.
Nonetheless, 57.7% (30/52) of patients had unfavorable responses to RUX and required additional treatment in this study. However, 53.3% (16/30) of these patients responded well to the subsequent chemotherapy regimen. These data suggest that RUX and traditional chemotherapy can be used as complementary regimens of frontline therapy, but further studies are warranted to identify the specific parameters of their application. On the other hand, 26.9% (14/52) of patients were refractory to both RUX and conventional chemotherapy, highlighting the urgent need for novel therapeutic approaches. The refractory patients were dominated by CAEBV-HLH, a challenging subgroup of HLH, especially in Asian countries. The persistent EBV infection in CAEBV patients leads to the sustained activation of HLH, which may explain its refractoriness. At present, the only effective treatment strategy to eradicate EBV-infected cells is allogeneic stem cell transplantation.28
This study has several limitations. First, the dosage of RUX is an important factor associated with treatment efficacy. However, because HLH usually deteriorates rapidly within a short time frame, patients in this study who had an insufficient response to RUX were immediately given additional therapy, lacking a dose-increase setting for longer tests. This premature combination with chemotherapy may affect the evaluation of the true efficacy of RUX monotherapy, especially for patients with HLH improvement. Meanwhile, the dose of RUX used in this study was rather low in light of the high levels of JAK-dependent cytokines in HLH. The lower RUX dose may be a possible reason for the lack of efficacy in certain patients. Although a previous study reported that the intermittent administration of high doses of RUX did not significantly improve HLH survival in murine HLH, with a narrow therapeutic window and potential toxicity,29 several other studies reported that this dose is effective and does not demonstrate toxicity.8,10,30,31 Furthermore, other publications have demonstrated that higher doses of RUX for various other diseases in humans, even when given with multiagent chemotherapy, can yield good clinical benefits and tolerance.20,32-34 Therefore, additional studies with high-dose RUX for HLH are required to confirm this possibility and guide RUX treatment for better clinical benefits. Second, there was an imbalance in the number of different underlying etiologies enrolled in this study, as EBV-HLH accounts for ∼60% of all cases of pediatric HLH at our center. Although this study describes a favorable efficacy profile, additional studies in larger patient cohorts are required. Third, critically ill patients and patients with severe CNS involvement were not included in this study. The efficacy and safety of RUX in these subgroups of patients should be elucidated in future studies.
In summary, our study demonstrates that RUX is an effective treatment option with good tolerance for pediatric HLH patients. The study provides support for the use of RUX as a frontline agent and stratification of patients based on early RUX response for subsequent therapy. The data of this study are valuable to future trials with RUX or other targeted agents.
Acknowledgments
The authors thank the patients and families who participated in this study.
This work was supported by the National Natural Science Foundation of China (no. 81800189), the Special Fund of the Pediatric Medical Coordinated Development Center of Beijing Municipal Administration (no. XTZD20180202), the Scientific Research Common Program of Beijing Municipal Commission of Education (no. KM201710025019), Beijing Municipal Administration of Hospitals’ Youth Programme (QML20181205), and National Science and Technology Key Projects (no. 2017ZX09304029003).
Authorship
Contribution: R.Z., Z.-G.L., and T.-Y.W. designed and supervised this study; Y.-Z.Z., H.-H.M., D.W., and A.W. recruited and treated the patients; L.C., W.-J.L., and C.-J.W. performed laboratory tests; Q.Z. and Y.-Z.Z. conducted the data analysis and wrote the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rui Zhang, Hematology Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, 56 Nan Lishi Rd, Beijing 100045, China; e-mail: ruizh1973@126.com; Zhi-Gang Li, Hematologic Disease Laboratory, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, 56 Nan Lishi Rd, Beijing 100045, China; e-mail: ericlzg70@hotmail.com; and Tian-You Wang, Hematology Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, 56 Nan Lishi Rd, Beijing 100045, China; e-mail: wangtianyou@bch.com.cn.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
Q.Z. and Y.-Z.Z. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal