In this edition of Blood, Tam et al report on the fixed-duration (FD) cohort of the CAPTIVATE study, which investigated ibrutinib plus venetoclax in treatment-naïve chronic lymphocytic leukemia (CLL).1
Currently approved treatment options in this setting include continuous monotherapy with a Bruton tyrosine kinase (BTK) inhibitor and FD venetoclax plus obinutuzumab. Preclinical data suggest synergy of BTK and BCL2 inhibitors, and single-center studies of ibrutinib plus venetoclax with or without obinutuzumab have shown promising efficacy.2,3 CAPTIVATE, however, is one of the most mature multicenter trials of this combination, and thus the field eagerly awaits updates from this study.
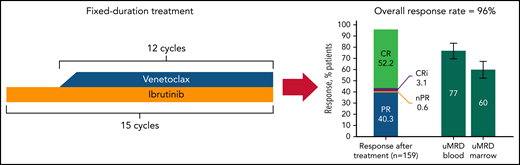
Although follow-up remains early with a median of only 27.9 months, the efficacy seen with this regimen on this trial is phenomenal, with a 56% rate of complete response and 76% and 62% of patients achieving undetectable minimal residual disease (uMRD) in the blood and bone marrow, respectively, using a cutoff of 1 x 10−4 cells (see figure). At 24 months, 95% of patients remained alive and free of progression. Significantly, outcomes were excellent for patients, even those with high genomic risk disease, such as abnormalities in TP53. Of note, there is a trend toward patients with unmutated IGHV having higher likelihood of uMRD. It will be important to see with longer follow-up whether this type of combination reverses the negative impact of unmutated IGHV on progression-free survival (PFS) observed with venetoclax and obinutuzumab but not with continuous BTK inhibitors.4,5 It also remains to be seen whether the negative impact of del17p will be mitigated by the inclusion of a BTK inhibitor in this FD regimen because, as with patients who have unmutated IGHV, the PFS is shorter in patients who have del17p and who receive initial treatment with FD venetoclax plus obinutuzumab compared to continuous BTK inhibitors.4,6,7 It is unclear whether it is the discontinuation of treatment or the target that accounts for this difference. These promising efficacy data with CAPTIVATE recapitulate what has been seen in smaller studies and provide continued evidence that ibrutinib plus venetoclax is an effective regimen for patients with treatment-naïve disease.
In the FD cohort of the CAPTIVATE study, treatment-naïve patients with CLL were treated with combined ibrutinib and venetoclax given for a total of 15 cycles of 28 days; treatment was then stopped. After completion of this regimen, the rate of complete remission was 52.2% with a high number of patients achieving uMRD in the blood (77%) or bone marrow (60%). CR, complete remission; CRi, CR with incomplete bone marrow recovery; nPR, nodular partial remission; PR, partial remission. The figure has been adapted from Figures 2A and 3A in the article by Tam et al that begins on page 3278.
In the FD cohort of the CAPTIVATE study, treatment-naïve patients with CLL were treated with combined ibrutinib and venetoclax given for a total of 15 cycles of 28 days; treatment was then stopped. After completion of this regimen, the rate of complete remission was 52.2% with a high number of patients achieving uMRD in the blood (77%) or bone marrow (60%). CR, complete remission; CRi, CR with incomplete bone marrow recovery; nPR, nodular partial remission; PR, partial remission. The figure has been adapted from Figures 2A and 3A in the article by Tam et al that begins on page 3278.
Interestingly, in the studies that have been performed using this doublet, including CAPTIVATE, it has not been demonstrated yet that depth of remission, either attainment of a complete response or bone marrow uMRD, correlates with PFS. This is in contrast to chemoimmunotherapy or venetoclax-antibody combinations, in which uMRD is correlated with longer PFS. Although this may simply be a function of short follow-up, it also raises the question of whether the biology of the CLL cells is altered by this type of combination. Certainly, these data call into question whether MRD-guided approaches, at least those focused on lengthening therapy for persistent MRD, are necessary to extend PFS. Future clinical trials will need to answer this question.
It is clear that additional follow-up is needed to determine the benefit of this regimen in terms of PFS. On the basis of response and MRD outcomes, it may be the best of both worlds with the limited duration of a venetoclax regimen coupled with the mitigation of poorer outcomes in high-risk patients receiving BTK inhibitors. That being said, when it comes to toxicity, this regimen also may be the worst of both worlds. Given the outstanding efficacy of both venetoclax and BTK inhibitors, selection of which regimen a physician or patient prefers is often based on the distinct toxicities of the agent. A regimen that contains both drugs does not avoid either agent’s adverse effects.
BTK inhibitors, particularly ibrutinib, have known associations with adverse cardiovascular events, including hypertension and arrhythmias. The 15-cycle limited treatment duration with the ibrutinib and venetoclax regimen studied in CAPTIVATE reduces the incidence of these adverse events, which increase with time receiving BTK inhibitors.8 However, cardiovascular events were still seen on this study. Attribution is always difficult, but 1 patient on the study experienced sudden death, as has been seen in other studies of ibrutinib. This risk, which seems lower in younger fit patients (like those included in this study) is important to consider in any regimen including BTK inhibitors, especially ibrutinib. In addition, 6% of patients experienced grade 3 to 4 hypertension and 4% had atrial fibrillation of any grade. The impact of atrial fibrillation on patient well-being is substantial because many will be symptomatic, will require treatment with medication, and will require anticoagulation therapy for stroke prophylaxis, which may further increase the risk of bleeding. Along those lines, the bleeding events known to occur with ibrutinib were seen in this study. Bleeding was reported in 59% of patients who were not receiving anticoagula tion or antiplatelet agents and increased slightly to 61% of those who were receiving those agents. Two percent (n = 3) of patients experienced major hemorrhages.
The inclusion of venetoclax required the standard dose ramp-up over 5 weeks to reduce the risk of tumor lysis syndrome with this agent. The all-oral nature of this regimen does limit infusion visits, but the visit burden needed to safely start venetoclax is still high. Even with the percentage of patients for whom hospitalization was recommended reduced to 18% after the 3-cycle ibrutinib lead-in, those who did not require a hospital stay still had the 5 weeks of outpatient visits to start venetoclax. Those lengthy and frequent visits remain a substantial challenge for some patients and clinics.
The results of the CAPTIVATE study as presented in the article by Tam et al are clearly outstanding. The prospect of offering this all-oral FD regimen to patients as an initial treatment is exciting, and the ability to achieve this level of uMRD with a short duration of treatment and durable remissions at early follow-up is a testament to how far the field has advanced. With additional follow-up and attention to adverse events and biologic characteristics of residual and recurrent CLL, it will hopefully become more clear which patients will derive the most benefit from this regimen.
Conflict-of-interest disclosure: K.A.R. received research funding from Genentech, AbbVie, Janssen, and Novartis; consulted for AstraZeneca, Innate Pharma, Pharmacyclics, Genentech, AbbVie, and BeiGene; and received travel funding from AstraZeneca. J.A.W. received research funding from Pharmacyclics, AbbVie, Janssen, Karyopharm, MorphoSys, and Schrodinger and has consulted for AbbVie, Pharmacyclics, Janssen, AstraZeneca, BeiGene, Genentech, Loxo Oncology, and Newave Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal