Abstract
When imatinib, the first tyrosine kinase inhibitor (TKI) developed for use in chronic myelogenous leukemia (CML), was approved in 2001, the treatment of this disease was forever changed. Significant reductions in the molecular burden of disease were seen with the first-generation TKI imatinib and, with the addition of dasatinib (2006), nilotinib (2007), bosutinib (2012), and ponatinib (2013), deeper and more rapid reductions were noted. Physicians could begin to tailor TKI therapy to individual patients, and patients who did not respond to or could not tolerate first-line therapy now had options. Importantly, the number of patients who developed accelerated or blast phase disease decreased dramatically. Research in CML continues to evolve; by presenting illustrative cases, this article reviews some of the newer aspects of clinical care in this disease. Updated information regarding bosutinib and asciminib, the latter currently in clinical trials, will be presented; bosutinib is of particular interest as the drug’s transit through the United States Food and Drug Administration highlights the question of what is considered optimal response to TKI therapy. The challenge of understanding the cardiac safety data of ponatinib and the unique dosing schedule based on individual response will be discussed. Lastly, two cases will focus on features of TKI treatment that, remarkably, have become part of the treatment algorithm: family planning for women with CML and stopping therapy after meeting a specific treatment milestone.
Introduction
Chronic myelogenous leukemia (CML) is a clonal myeloproliferative neoplasm characterized by an unregulated expansion of myeloid cells in the bone marrow. In 1960, Peter Nowell and David Hungerford first suggested a causal relationship between a “minute chromosome” and chronic granulocytic leukemia, as CML was called at the time.1 Subsequent to this, Dr. Janet Rowley determined that the “minute chromosome” was a shortened chromosome 22 that developed following a reciprocal translocation between chromosome 9 and 22,2 specifically the ABL1 gene on chromosome 9 was inserted in the BCR region of chromosome 22. This translocation generated a fusion oncoprotein, BCR-ABL1, which produced a dysregulated tyrosine kinase.3 This, in turn, led to the development of the first oral tyrosine kinase inhibitor (TKI), imatinib.4
The last review of chronic-phase CML in Blood was in 20125 and since then, numerous additions and modifications have been integrated into therapeutic approaches for this disease. Bosutinib, a dual Abl and Src inhibitor, was approved by the US Food and Drug Administration (FDA) in 2012 for second-line use,6-8 and by the European Medicines Agency in 2013. Bosutinib was then approved for first-line use in the United States in 2017 and by the European Medicines Agency in 2018. Ponatinib, which has activity across disease stages and mutations including T315I,9 was approved in the United States in 2012 for second-line use, temporarily suspended from commercial distribution because of heightened risk of vascular toxicity, and reapproved for patients for whom no other TKI was indicated in 2013. In Europe, ponatinib was approved in 2013 for patients who were resistant or intolerant to dasatinib or nilotinib and for whom imatinib was not clinically appropriate. Recent preliminary data suggest that a novel dosing schedule based on individual patient response may decrease the incidence of vascular toxicity.10 Based on these data, in 2020 the FDA again revised the indication to include treatment of chronic-phase patients with resistance or intolerance to at least 2 prior TKIs. A new TKI, asciminib (ABL-001), designed to block the myristoyl binding site on the BCR-ABL1 kinase, has shown significant activity in heavily treated chronic-phase patients, including those with the T315I mutation.11,12 Family planning is now part of the conversation, and stopping therapy is now incorporated into treatment options for patients who reach specific therapeutic milestones. These advances have had a major impact on patients who can, in most instances, look forward to a normal life span, and to clinicians, who now have more options for starting and stopping treatment.
Here, 5 cases that provide examples of how these new developments have been incorporated into clinical practice are presented.
Ponatinib vascular toxicity reexamined with a new dose-response schedule recommended
Patient 1
The patient is a 45-year-old woman with chronic-phase CML diagnosed in January 2018 and begun on dasatinib 100 mg daily. The dose of dasatinib was reduced to 70 mg daily because of persistent thrombocytopenia (platelets <50 000/μL) in April 2018. In August 2018, her polymerase chain reaction (PCR) using international scale (IS) was 15%IS despite being compliant with daily dosing. She was changed to nilotinib 300 mg twice daily but in November 2018, her PCR was 52%IS. ABL1 mutation studies showed the presence of the T315I mutation and she was changed to ponatinib 45 mg daily. She was also begun on a statin for hyperlipidemia, an antihypertensive medication, and low-dose aspirin. Based on preliminary results of the Optimizing Ponatinib Treatment in CML (OPTIC) trial (NCT02467270),10 I reduced her dose of ponatinib to 15 mg daily after she achieved a complete cytogenetic response (CCyR), defined as a PCR <1% (see Table 1 for definitions).13 She was seen by a member of our bone marrow transplant team but had no sibling donor and no donor identified through the International Registry. A cord blood transplant could be considered but for now, I chose to continue her on ponatinib. In October 2020, the patient’s PCR was 0.19%IS.
Definition of molecular response to TKI therapy per International Scale (IS)*
| PCRIS . | Response . |
|---|---|
| ≤1.0% | Complete cytogenetic response (CCyR) |
| ≤0.1% | Major molecular response (MMR) |
| ≤0.01% | Deep molecular response4 (MR4) |
| ≤0.0032% | Deep molecular response4.5 (MR4.5) |
| ≤0.001% | Deep molecular response5 (MR5) |
| PCRIS . | Response . |
|---|---|
| ≤1.0% | Complete cytogenetic response (CCyR) |
| ≤0.1% | Major molecular response (MMR) |
| ≤0.01% | Deep molecular response4 (MR4) |
| ≤0.0032% | Deep molecular response4.5 (MR4.5) |
| ≤0.001% | Deep molecular response5 (MR5) |
As determined by reverse transcriptase quantitative polymerase chain reaction.
Ponatinib has potent activity against native and mutant BCR-ABL1, including the T315I mutation and, until recently, was the only compound effective against this highly resistant mutation.9,14-16 The phase 2 Ponatinib Ph+ ALL and CML Evaluation (PACE; NCT01207440) trial included heavily treated patients with chronic phase who were either resistant to or intolerant of dasatinib or nilotinib (n = 270), in accelerated phase (n = 85), blast phase (n = 62), or had Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (n = 32).15 In the group of chronic-phase patients, 27% of resistant and 56% of intolerant patients achieved a major molecular response (MMR, defined as a PCR <0.1%IS) with ponatinib at a dose of 45 mg daily. Of note, T315I was not a predictor of response. At a median follow-up time of 57 months, the estimated likelihood of a sustained response was 91% at 12 months and the rate of congestive heart failure, the only vascular toxicity reported, was 3%, within the range of the other second-generation TKIs dasatinib17 and nilotinib.18
The drug was then fast-tracked by the FDA in December 2012, and received accelerated approval for second-line use in patients whose disease had progressed on or were intolerant to prior TKIs, at the dose of 45 mg daily. However, less than 1 year later, in October 2013, because of concerns over vascular occlusive risks, the FDA temporarily suspended the drug from commercial distribution in the United States. Ponatinib was reapproved for marketing 2 months later in December 2013, with revised prescribing information that included a boxed warning of vascular risks. Importantly, the package insert in 2013 did not give clear guidance on dose reductions, citing that the optimal dose was not identified and only suggested considering dose reduction upon achieving a major cytogenetic response (0%-35% Ph+ metaphases) for chronic and accelerated phase patients.
Interpreting the cardiovascular toxicity of ponatinib in the PACE trial has proven to be a challenge. When calculating the rate of vascular occlusive events, the FDA used ∼400 preferred terms based on the Medical Dictionary for Regulatory Affairs v20.119 to identify arterial occlusive events (AOEs) in the PACE trial.15 Captured events included those based on symptoms thought to be related to vascular side effects; the results of this review suggested that there might be a dose-toxicity relationship. An independent review committee composed of 3 cardiologists, 1 neurologist, and 1 vascular specialist, all of whom were involved in cardiology endpoint adjudication, was established by the drug’s sponsor, Ariad (Takeda) Pharmaceuticals, to provide clarity and more closely analyze the vascular toxicity data. This review group reviewed the same PACE database but used event definitions provided by the Cardiovascular Endpoints Used in Clinical Trials designed by the American College of Cardiology.20 Events included both AOEs (sudden cardiac death, myocardial infarction, stroke, peripheral vascular disease, or hospitalization for unstable angina) and venous thrombotic events (venous thrombosis, pulmonary embolism, superficial vein thrombosis, deep vein thrombosis) or other AOEs or venous thrombotic events that required vascular intervention. Table 2 shows the comparison of results when individual AOEs were analyzed by both Medical Dictionary for Regulatory Affairs and Cardiovascular Endpoints definitions.21 Most categories are in close agreement but in 2, angina and intermittent claudication, the incidence is 0% using the Cardiovascular Endpoints criteria. Of note, most oncologists are not familiar with either of these 2 methodologies because the National Cancer Institute Common Terminology Criteria for Adverse Events is the one most frequently used in clinical oncology trials.22
Most common AOEs: PACE trial (N = 449 patients)
| Event, no. (%) . | Preadjudication* . | Adjudicated† . |
|---|---|---|
| Angina pectoris | 28 (6%) | 0 |
| Peripheral arterial occlusive disease | 22 (5%) | 19 (4%) |
| Intermittent claudication | 11 (2%) | 0 |
| Myocardial infarction | 18 (4%) | 10 (2%) |
| Coronary artery disease | 14 (3%) | 7 (2%) |
| Peripheral arterial stenosis | 10 (2%) | 8 (2%) |
| Cerebrovascular | 8 (2%) | 7 (2%) |
| Carotid artery stenosis | 7 (2%) | 7 (2%) |
| Peripheral arterial occlusion | 7 (2%) | 7 (2%) |
| Event, no. (%) . | Preadjudication* . | Adjudicated† . |
|---|---|---|
| Angina pectoris | 28 (6%) | 0 |
| Peripheral arterial occlusive disease | 22 (5%) | 19 (4%) |
| Intermittent claudication | 11 (2%) | 0 |
| Myocardial infarction | 18 (4%) | 10 (2%) |
| Coronary artery disease | 14 (3%) | 7 (2%) |
| Peripheral arterial stenosis | 10 (2%) | 8 (2%) |
| Cerebrovascular | 8 (2%) | 7 (2%) |
| Carotid artery stenosis | 7 (2%) | 7 (2%) |
| Peripheral arterial occlusion | 7 (2%) | 7 (2%) |
Categorization of AOEs based on Medical Dictionary for Regulatory Activities (MedDRA) preferred terms related to vascular ischemia or thrombosis used by the FDA.
Categorization of AOEs based on cardiovascular endpoints used in clinical trials used by the Independent Adjudication Committee.
To establish whether there was a dose-toxicity relationship with ponatinib, the OPTIC trial was designed, the aim of which was to ascertain whether the dose of ponatinib could be decreased at a specified response milestone without loss of response, and whether the ponatinib dose was related to vascular toxicity.10 The specific milestone to be reached was CCyR (PCR ≤1%) by 12 months. Chronic-phase patients resistant or intolerant to 2 or more TKIs were randomized to ponatinib at a starting dose of 45 mg (cohort A), 30 mg (cohort B), or 15 mg (cohort C) daily. Cohorts A and B had the ponatinib dose decreased to 15 mg if CCyR was achieved at any time during the study. A total of 283 patients were randomized to cohorts A/B/C: 94/95/94 patients. All 3 groups contained patients who had received 3 or more prior TKIs (cohorts A/B/C: 53%/60%/51%).
Preliminary results were presented at the 2020 American Society of Hematology Annual Meeting.10 Interim analysis was performed at a median follow-up time of 21 months and showed that in a largely resistant population (99% resistant) in which the majority of patients (62%) failed to achieve a response greater than a complete hematologic response (normal complete blood cell count) on prior therapy, the rate of achieving a CCyR was highest in cohort A, the 45 mg starting dose: cohorts A/B/C: 39%/27%/27%. In all categories of ABL1 mutations, the rate of CCyR by 12 months was again highest in cohort A (cohorts A/B/C: 59%/30%/21%), with the most striking difference in the T315I mutation group (cohorts A/B/C: 60%/25%/6%). Patients with no mutations or mutations other than T315I had small differences, but the outcomes still favored the 45-mg starting dose. The same group of reviewers who reviewed the PACE trial and who were now blinded to dose reviewed the treatment-emergent AOEs in the OPTIC trial (AOEs A/B/C: 5%/4%/1%). These preliminary data suggest that with this individual dose-adjustment schedule, it may be possible to achieve a deep molecular response, even in heavily treated patients, and at the same time avoid the potential cardiac toxicity of this active TKI.
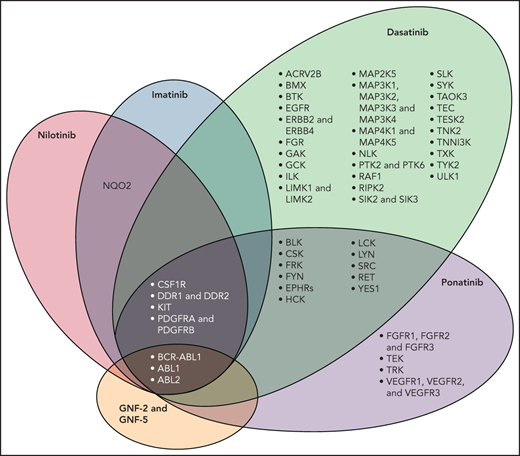
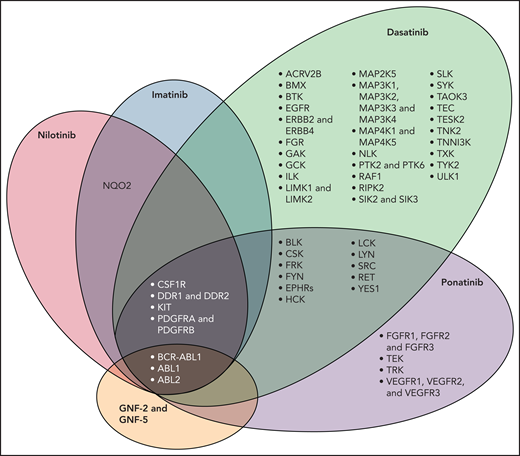
In addition to BCR-ABL1, TKIs have activity against a number of kinases that have a role in vascular biology such as vascular endothelial growth factor receptors 1-3, platelet-derived growth factor receptors A and B (PDGFR A/B) and fibroblast growth factor receptor. Figure 123 shows a schematic representation of the molecular domains of each of the TKIs with the exception of bosutinib. In addition to inhibiting BCR-ABL1, all TKIs inhibit PDGFR A and PDGFR B; ponatinib has a broader domain which includes vascular endothelial growth factor receptors 1-3. VEGF helps maintain endothelial cell integrity by activating survival and anti-apoptotic signaling; it also increases bioavailability of vasodilators nitrous oxide and prostacyclin.24 Inhibition of VEGF thus has the potential to interfere with regenerative capabilities of endothelial cells as well as induce defects in the endothelium that can lead to both bleeding and thrombosis.24,25 Data also suggest that inhibitors of the vascular signaling pathway can form immune complexes that activate platelets and induce thrombosis.26,27
Molecular domain structures of ABL1 family kinases.23 Specificity of selected ABL tyrosine kinase inhibitors is shown. The kinase selectivity profiles for imatinib, nilotinib, and dasatinib were generated on the basis of binding of cellular kinases to inhibitors immobilized on solid support matrices; ponatinib sensitive kinases were identified by in vitro kinase assays; shown are targets with half maximal inhibitory concentration values less than 20 nM. Abbreviations: AVR2B, activin receptor type 2B; BLK, B lymphoid tyrosine kinase, CSF1R, macrophage colony stimulating factor 1 receptor; EGFR, epidermal growth factor receptor; EPHRs, ephrin receptors; FGFR, fibroblast growth factor receptor; FRK, fyn-related kinase; GAK, cyclin-G-associated kinase; GCK, glucokinase; ILK, integrin-linked protein kinase; LIMK, Lim domain kinase; NLK, nemo-like kinase; NQO2, NAD(p)H dehydrogenase, quinone 2; PDGFR, platelet-derived growth factor receptor; PTK, protein tyrosine kinase; RIPK2, receptor-interacting serine/threonine-protein kinase 2; SIK, salt-inducible kinase; SLK, STE20-like serine/threonine-protein kinase; TAOK3, TAO kinase 3; TESK2, dual specificity testis-specific protein kinase 2; VEGFR, vascular endothelial growth factor receptor. (Used with permission.)
Molecular domain structures of ABL1 family kinases.23 Specificity of selected ABL tyrosine kinase inhibitors is shown. The kinase selectivity profiles for imatinib, nilotinib, and dasatinib were generated on the basis of binding of cellular kinases to inhibitors immobilized on solid support matrices; ponatinib sensitive kinases were identified by in vitro kinase assays; shown are targets with half maximal inhibitory concentration values less than 20 nM. Abbreviations: AVR2B, activin receptor type 2B; BLK, B lymphoid tyrosine kinase, CSF1R, macrophage colony stimulating factor 1 receptor; EGFR, epidermal growth factor receptor; EPHRs, ephrin receptors; FGFR, fibroblast growth factor receptor; FRK, fyn-related kinase; GAK, cyclin-G-associated kinase; GCK, glucokinase; ILK, integrin-linked protein kinase; LIMK, Lim domain kinase; NLK, nemo-like kinase; NQO2, NAD(p)H dehydrogenase, quinone 2; PDGFR, platelet-derived growth factor receptor; PTK, protein tyrosine kinase; RIPK2, receptor-interacting serine/threonine-protein kinase 2; SIK, salt-inducible kinase; SLK, STE20-like serine/threonine-protein kinase; TAOK3, TAO kinase 3; TESK2, dual specificity testis-specific protein kinase 2; VEGFR, vascular endothelial growth factor receptor. (Used with permission.)
Because smoking, diabetes, hypertension, hyperlipidemia, obesity, and a sedentary lifestyle all increase the risk for TKI-induced cardiovascular toxicity, it is extremely important that risk reduction education be provided to all CML patients: this could include enhancing cooperation with the patient’s internist, or referral to a cardiologist for risk reduction strategies.
I attempt to minimize potential cardiac risk factors for all patients who begin TKI therapy. I make sure all patients have a fasting lipid screen and glucose, blood pressure measurements, and a baseline electrocardiogram. The role of a resting cardiac echocardiogram is debatable, but I usually obtain one, especially if starting dasatinib. I also obtain a baseline chest radiograph. If a patient is currently smoking, I always discuss the importance of stopping and frequently refer to one of many online programs or the one sponsored by the American Lung Association. I emphasize the importance of exercise and, when necessary, weight control. I usually refer management of hypertension, if present, to the patient’s general medical doctor. Although there are no data to support the use of low-dose aspirin in patients taking ponatinib, I usually add this unless there is a contraindication. I should note that apart from the use of low-dose aspirin, the same recommendations would be made regardless of the TKI started. Lastly, I would recommend routine bone density exams. We have previously shown that imatinib—and presumably other TKIs—can inhibit osteoblast and to a lesser degree osteoclast activity,28 which has led to premature osteopenia and osteoporosis in both men and women.29
The patient’s most recent PCR from October 2020 was 0.19%IS and, based on the OPTIC trial data,10 I decreased her ponatinib dose to 15 mg daily and continue to monitor her PCR every 3 months. Her cholesterol and blood pressure are now controlled and she continues to work on weight control.
However, the optimal treatment strategy for this 45-year-old patient with a T315I mutation who will likely require lifelong ponatinib is not clear, which is why I requested a consultation with a member of our bone marrow transplant team. Historically, preferred donors for patients with hematologic malignancies have been HLA-matched, but stem cell transplant (SCT) outcomes using alternative donors (cord blood, haploidentical) have improved over the past decade. To my knowledge, there are no studies that have compared long-term ponatinib with any type of SCT in high-risk patients such as the one described here. In the absence of such data, the care-giver and the patient must weigh potential risks and benefits such as vascular toxicity with ponatinib and chronic graft-versus-host disease and/or long-term immune suppression with SCT. Fortunately, peripheral blood PCR testing can almost always allow for early detection of disease progression, although there is the rare patient who transforms to advanced phase disease very quickly. This patient and I discussed at length the significance of the T315I mutation, the potential risks of vascular events with ponatinib, and the side effects of cord blood transplant within her age group, and we both felt comfortable with continuing ponatinib with frequent PCR monitoring.
Family planning for a young woman with CML
Patient 2
The patient is a 35-year-old woman diagnosed with chronic-phase CML in December 2005 at age 19 and begun on imatinib 400 mg daily. In June 2006, her PCR was 7%IS and her dose of imatinib was increased to 600 mg daily. In December 2006, her PCR was 0.08%IS and serial PCRs have remained within that range. In 2019, at age 33, the patient wanted to start a family. Her PCR was 0.09%IS at the time. The patient and her partner were referred to a nearby in vitro fertilization (IVF) center. She stopped imatinib for 2 months and had 8 oocytes harvested and fertilized, but only 2 embryos were viable for implantation. The decision was made to proceed with a surrogate pregnancy and the patient restarted imatinib 600 mg daily. Nine months later, 2 healthy baby girls were delivered. The patient’s most recent PCR in November 2020 was 0.06%IS.
Results from the EUTOS population-based study suggest that ∼37% of patients at the time of CML diagnosis are of reproductive age, making it important for clinical care teams to be aware of options for family planning.30 Data on the effect of TKIs on ovarian function are limited, mostly to case reports and small series. In one, a 15-year-old young woman with CML who began imatinib underwent oocyte retrieval at age 17 while taking the drug; only 8 oocytes were retrieved.31 Follicular fluid contained measurable levels of imatinib consistent with an equilibrium phase between plasma and follicular compartments. Imatinib was then stopped for 2 months and she underwent a second retrieval: this time, 43 oocytes were retrieved and no imatinib was detected in the follicular fluid, suggesting that imatinib may have had a direct effect on oocyte suppression.
There have been limited studies of fertility in men taking TKIs; however, the general consensus is that there appears to be no deleterious effect.32-34 In a review of the Bristol Myers Squibb dasatinib pharmacovigilance database, 30 of 33 infants (91%) fathered by men taking dasatinib were normal at birth.33 Similarly, neither the risk of miscarriage nor the fetal malformation rate was higher than average in the pregnant partners of men taking imatinib.34
It is strongly suggested that women not become pregnant while taking any of the TKIs. Embryo development occurs between weeks 3 and 10 of gestation with most organogenesis occurring between weeks 5 and 8, making this the most sensitive time period for fetal damage.35 The mechanisms responsible are not always well defined, but damage may occur from direct cellular damage, vascular effects, or alterations in hemodynamic flow between the placenta and the embryo. TKI therapy during pregnancy has been linked to fetal malformations, although there does not appear to be a defining pattern. Pye et al36 reported the largest series collected from the Hammersmith Hospital in London, the M.D. Anderson Cancer Center in Houston, and Novartis Pharmaceuticals. Fetal outcomes were reported in 125 pregnancies. Normal live births were reported in 63 pregnancies (50%), 28 women underwent elective terminations (3 following identification of fetal abnormalities), 18 pregnancies ended in spontaneous miscarriages (14%), 8 babies were born with congenital abnormalities (6%), and there was 1 stillbirth. Cortes et al reported similar outcomes in women taking dasatinib using the Bristol Myers Squibb database through 2013.32
Although it is generally agreed that TKI therapy should be stopped immediately once a woman suspects she may be pregnant, there are no published data regarding how long a woman should be off therapy before attempting a natural pregnancy or an IVF attempt. In this regard, each patient should be individually assessed and counseled with regard to implications of losing response and risk of disease progression.
Management of women who are diagnosed with CML during pregnancy is largely based on retrospective reports.37,38 Leukapheresis and/or plateletpheresis can be used during any trimester to keep the white blood cell and/or platelet count <100 000/μL or platelet count <500 000/μL, respectively, although no guidelines exist for what hematologic parameters should be used. Both pegylated interferon-α and standard interferon-α have been used safely during pregnancy, although the data are from women with essential thrombocytosis.39,40 Hydroxycarbamide (hydroxyurea) should be avoided in all trimesters because it has been linked to intrauterine fetal deaths, premature delivery, and preeclampsia.37 More detailed discussions of therapeutic options have been described elsewhere.37,41,42
This patient brings out a number of interesting points. According to a comprehensive review of fertility published by Schattman,43 the likelihood of natural conception in women over the age of 35 is in the range of 54%, whereas that of women younger than 31 is 74%, suggesting that the “watershed” years for many women are in their early 30s. Given that there is no established time recommended before stopping TKI therapy before attempting natural conception or oocyte retrieval, and a case report suggesting that imatinib can inhibit oogenesis,31 I suggested that this particular patient not stop imatinib and attempt a natural conception, as she was in the “older” age group and had been on therapy for 14 years with the quality of her oocytes an unknown factor, but did suggest an IVF attempt. A second important point is that although we now have 3 other TKIs that are approved for first-line use, we tend to forget that responses can be seen with higher doses of imatinib, in the 600- to 800-mg dose range.42 This can be an option for patients who have limited insurance choices or in settings where other TKIs are not easily available. Lastly, IVF is not within everyone’s reach: this is an expensive procedure, in the range of $12 000 to $14 000 (US) per cycle, not including the cost of drugs, and many insurance companies do not cover this procedure.41 It is also important to recognize that religious and legal restrictions in some countries do not permit more oocytes fertilized than the number of embryos a woman is willing to have transferred in a single IVF cycle. In the United States, not all states allow surrogacy.
How approval of bosutinib highlighted the question of optimal response to TKI therapy
Patient 3
This patient is a 62-year-old man with chronic-phase CML diagnosed in October 2016. He was begun on imatinib 400 mg daily but in April 2017, his PCR was 4%IS. ABL1 mutation studies were negative. Bosutinib 400 mg was begun and by November 2017 his PCR was 0.49%IS. Over the last 3 years, his PCR has remained between 0.1% and 0.9%IS; his most recent PCR in November 2020 was 0.29%IS.
Bosutinib is a dual Src/Abl inhibitor with more potent activity in CML cell lines than imatinib and less inhibitory activity against c-KIT and PDGFR, which are associated with side effects reported with other TKIs.6-8 The primary aim in the initial phase 2 trial (Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia [BELA] trial), which randomized 502 newly diagnosed chronic-phase CML patients to either bosutinib 500 mg daily or imatinib 400 mg daily, was to determine whether there was a significant difference in the incidence of CCyR (PCR <1.0%IS) after 12 months of therapy.7 The results showed no difference between bosutinib and imatinib, 70% vs 68%, P = .601, respectively. However, there was a significant difference in MMR (PCR <0.1%IS) at 12 months: 41% vs 27%, P ≤ .001. Nonetheless, as the primary endpoint of the study was CCyR at 12 months, and as this endpoint was not met, the sponsor could not move forward with FDA approval.
This brought up the question of what is considered the optimal response to TKI therapy: is it achievement of a CCyR or an MMR? Pfizer, the company that developed the drug, then designed a second randomized trial in newly diagnosed chronic-phase CML patients with the same trial design as the BELA trial, but this time the primary endpoint was MMR at 12 months; the dose of bosutinib was also reduced to 400 mg daily (Bosutinib Trial in First Line Chronic Myelogenous Treatment [BFORE] trial).8 In this large trial (536 patients), bosutinib again demonstrated a higher MMR compared with imatinib at 12 months, 47% vs 36% (P = .02), and the drug was approved by the FDA for first-line use in 2018. The MMR rates for bosutinib (41% in the BELA trial, 47% in the BFORE trial) are similar to the MMR rates with dasatinib 46%17 and nilotinib 43%.18
There are 2 main treatment guidelines for use in patients with CML, the National Comprehensive Cancer Network (NCCN) guidelines42 and the European LeukemiaNet (ELN).44 Both use similar response categories: the ELN uses “optimal,” “warning,” and “failure,” whereas the NCCN uses “TKI-sensitive disease,” “possible TKI resistance,” and “TKI-resistant.” Both the “warning” and “possible TKI resistance” categories alert the clinician that a change in TKI might be warranted. However, these categories are not perfectly aligned. The ELN considers patients who have a PCR between 0.1% to 1%IS at 12 months or later to be in the “warning” category (Table 3), whereas the NCCN does not (Table 4). The NCCN decision is based on data from trials that show an MMR (PCR <0.1%IS) at 12 or 18 months of therapy does not confer any survival benefit compared with a CCyR (PCR <1.0%).45,46 In the Jabbour study,45 167 newly diagnosed chronic-phase patients were treated with either second-generation dasatinib or nilotinib; 155 patients (93%) achieved a CCyR and of these, 146 patients (87%) achieved an MMR. There was no difference in survival between the 2 groups. However, the NCCN does clarify that the goal of reaching MMR should be considered if the patient would like to consider stopping therapy or for women who would like to consider pregnancy.42
Milestones for treating CML expressed as BCR-ABL1IS per LeukemiaNet44
| Month . | Optimal . | Warning . | Failure . |
|---|---|---|---|
| 3 | ≤10% | >10% | >10% if confirmed |
| 6 | ≤1% | >1%-10% | >10% |
| 12 | ≤0.1% | >0.1%-1% | >1% |
| Anytime | ≤0.1% | >0.1%-1% | >1% |
| Loss of <0.1% |
| Month . | Optimal . | Warning . | Failure . |
|---|---|---|---|
| 3 | ≤10% | >10% | >10% if confirmed |
| 6 | ≤1% | >1%-10% | >10% |
| 12 | ≤0.1% | >0.1%-1% | >1% |
| Anytime | ≤0.1% | >0.1%-1% | >1% |
| Loss of <0.1% |
Milestones for treating CML expressed as BCR-ABL1IS per NCCN42
| Month . | >10% . | >1%-10% . | >0.1%-1% . | ≤0.1% . |
|---|---|---|---|---|
| 3 | Possible TKI resistant | TKI sensitive | TKI sensitive | TKI sensitive |
| 6 | TKI resistant | TKI sensitive | TKI sensitive | TKI sensitive |
| 12 | TKI resistant | Possibly TKI resistant | TKI sensitive* | TKI sensitive† |
| Month . | >10% . | >1%-10% . | >0.1%-1% . | ≤0.1% . |
|---|---|---|---|---|
| 3 | Possible TKI resistant | TKI sensitive | TKI sensitive | TKI sensitive |
| 6 | TKI resistant | TKI sensitive | TKI sensitive | TKI sensitive |
| 12 | TKI resistant | Possibly TKI resistant | TKI sensitive* | TKI sensitive† |
If treatment goal is long-term survival, >0.1% to 1% optimal.
If treatment goal is treatment-free remission, ≤0.1% is optimal.
This patient often serves as a point of discussion with our fellows: should he remain on bosutinib as recommended by NCCN guidelines (“TKI-sensitive disease”) or should he be changed to another second-generation TKI as recommended by the ELN (“warning category”). Because he was tolerating bosutinib very well, and as he has stated many times that he would not feel comfortable stopping therapy even if his PCR were lower, I chose to continue bosutinib given the previously noted studies that show no difference in overall survival between patients who achieve a CCyR and an MMR.
Use of asciminib in a patient with hematologic intolerance to dasatinib, imatinib, ponatinib, and bosutinib
Patient 4
The patient is a 69-year-old man with chronic-phase CML diagnosed in October 2015. He began dasatinib 100 mg daily, developed thrombocytopenia (platelets <50 000/μL), and was changed to imatinib in 2016, but still had persistent thrombocytopenia. Multiple mutation studies were negative. He was changed to bosutinib in March 2017, then ponatinib without improvement in his platelet count. In July 2017, his PCR was 17%IS and, later that month, he began asciminib 40 mg daily, then called by its investigational name, ABL-001, as part of the phase 1 clinical trial. His platelets increased to the range of 100 000/μL and his most recent PCR from December 2020 was 0.0024%IS.
Currently approved TKIs target the ATP-binding site of BCR-ABL1; however, other sites can also regulate ABL1 activation. For example, upon BCR-ABL1 fusion, autoinhibition of the ABL1 myristoylated N terminus is lost, resulting in ABL1 kinase activation.11 Asciminib was designed to bind to the myristoyl site, thereby restoring inhibition of the BCR-ABL1 kinase.11 A phase 1 dose-escalation study published by Hughes et al12 evaluated the drug in 141 chronic-phase patients, 105 of whom (70%) had received treatment with at least 3 prior TKIs. The dose ranged from 10 mg to 200 mg once or twice daily and, although a maximum tolerated was not identified, the dose of 40 mg twice daily was chosen as the incidence of pancreatitis was noted to be 3% at higher doses. An MMR was achieved or maintained by 12 months in 44 patients of 91 patients (54%) who could be evaluated including 8 of 14 patients (57%) who were either resistant or intolerant to ponatinib, and 5 patients (28%) who had the T315I mutation at baseline. Common side effects included malaise, headache, arthralgias, and thrombocytopenia. Ischemic stroke and peripheral arterial occlusive disease were each reported in 2 patients, both of whom had underlying cardiovascular disease.
Cortes et al recently provided more detailed information on 52 patients with the T315I mutation treated at a dose of 200 mg twice daily: 23 of the 49 (47%) of evaluable patients not in an MMR at baseline achieved an MMR.47 In the 28 patients who had previously received ponatinib, the estimated rate of MMR at 60 weeks was 32%. In addition, Hochhaus et al48 reported preliminary results of a phase 3 trial (the ASCEMBL trial) that randomized patients in chronic phase who had received >2 prior TKIs to either asciminib 40 mg twice daily or bosutinib 500 mg daily. CCyR rates at 24 weeks were 40% on the asciminib arm and 24% on the bosutinib arm, and the proportion of patients who discontinued treatment was 5% on the asciminib arm and 21% on the bosutinib arm. Decisions regarding whether to use ponatinib or asciminib, assuming the drug is approved by the FDA, for patients for patients with the T315I mutation or for patients not responding to at least second-generation TKIs, must await the final results of this trial.
The patient had an excellent response to asciminib. Nonetheless, I recommended that he be seen by our Bone Marrow Transplant Service for an opinion; the best donor option was his haploidentical son. Decision-making for this patient was done along the lines of the decision-making for patient 1, although in this setting, the patient did not have the T315I mutation and the TKI was asciminib, which has not yet been approved by the FDA. A haploidentical transplant could be an option, but given the deep response the patient achieved with asciminib (MR4.5), that he has maintained this response for more than 3 years, and his age (69), I recommended he continue asciminib. If his disease does progress, his son could presumably be readily available as a donor.
Stopping TKI therapy followed by the withdrawal syndrome
Patient 5
The patient is a 55-year-old man diagnosed with chronic-phase CML in February 2009 and begun on imatinib 400 mg daily. In August 2009, his PCR was 12%IS and he was changed to dasatinib 100 mg daily. ABL1 mutation studies were negative. His PCR decreased, and in June 2013, it was 0.001%IS. His PCR remained in that range and, after discussion, he stopped dasatinib in October 2017. One month later, he developed diffuse myalgias and bone pain.
The first trial that conclusively demonstrated the safety of stopping TKI therapy was the STIM1 trial published in 2008: 100 patients on imatinib who were in an MR5 for at least 2 years stopped therapy; at a median follow-up of 77 months, 38% of patients remained in a treatment-free remission.49 Since then, more than a dozen trials have stopped imatinib,50-54 dasatinib,55-59 and nilotinib.59-61 As shown in Table 5, despite the differences in the depth of response required before stopping therapy (MR4, MR4.5, MR5) and the length of time required to maintain that response before stopping therapy which ranged from 12 to 24 months, treatment-free remission rates remain fairly constant at around 50%.42,44 Notably, most recurrences occur within the first year after stopping therapy; however, almost all patients regain their response after restarting.
Summary of limited long-term follow-up data from the TKI discontinuation trials42
| Trial . | Treatment before discontinuation . | No. of patients . | Depth and duration of MR required for discontinuation . | Trigger to resume TKI therapy . | Median follow-up . | Treatment-free remission rate . |
|---|---|---|---|---|---|---|
| STIM149 | Imatinib ± interferon | 100 | MR5 for at least 2 y | Loss of MR5 | 77 mo | 38% at 60 mo |
| TWISTER50 | Imatinib ± interferon | 40 | MR4.5 for at least 2 y | Loss of MR5 | 103 mo | 45% at 8 y |
| HOVON51 | Imatinib ± cytarabine | 15 | MR4.5 for at least 2 y | Loss of MR4.5 | 36 mo | 33% at 24 mo |
| A-STIM52 | Imatinib ± interferon | 80 | MR5 for at least 2 y | Loss of MMR | 31 mo | 61% at 36 mo |
| ISAV53 | Imatinib (after failure of interferon or hydroxyurea) | 108 | CMR for at least 18 mo | Loss of MMR | 36 mo | 52% at 36 mo |
| KID54 | Imatinib ± interferon | 90 | MR4.5 for at least 2 y | Loss of MMR | 27 mo | 59% at 24 mo |
| Stop 2G-TKI55 | Dasatinib/nilotinib (first or second line) | 60 | MR5 for at least 24 mo | Loss of MMR | 47 mo | 54% at 48 mo |
| DASFREE56 | Dasatinib (first or second line) | 84 | MR4.5 for 12 mo | Loss of MMR | 2 y | 46% at 24 mo |
| ENESTFreedom60 | Nilotinib (first line) | 190 | MR4.5 for 12 mo | Loss of MMR | 96 wk | 49% at 96 wk |
| ENESTop study61 | Nilotinib (second line) | 126 | MR5 for at least 2 y | Loss of MMR | 96 wk | 53% at 96 wk |
| DADI57 | Dasatinib (first line) | 68 | MR4.5 for at least 24 mo | Loss of MMR | 23 mo | 55% at 6 mo |
| DADI58 | Dasatinib (second line) | 63 | MR4 for at least 12 mo | Loss of MR4 | 44 mo | 44% at 36 mo |
| EURO-SKI59 | Any TKI | 758 | MR4 for at least 1 y | Loss of MMR | 27 mo | 50% at 24 mo |
| Trial . | Treatment before discontinuation . | No. of patients . | Depth and duration of MR required for discontinuation . | Trigger to resume TKI therapy . | Median follow-up . | Treatment-free remission rate . |
|---|---|---|---|---|---|---|
| STIM149 | Imatinib ± interferon | 100 | MR5 for at least 2 y | Loss of MR5 | 77 mo | 38% at 60 mo |
| TWISTER50 | Imatinib ± interferon | 40 | MR4.5 for at least 2 y | Loss of MR5 | 103 mo | 45% at 8 y |
| HOVON51 | Imatinib ± cytarabine | 15 | MR4.5 for at least 2 y | Loss of MR4.5 | 36 mo | 33% at 24 mo |
| A-STIM52 | Imatinib ± interferon | 80 | MR5 for at least 2 y | Loss of MMR | 31 mo | 61% at 36 mo |
| ISAV53 | Imatinib (after failure of interferon or hydroxyurea) | 108 | CMR for at least 18 mo | Loss of MMR | 36 mo | 52% at 36 mo |
| KID54 | Imatinib ± interferon | 90 | MR4.5 for at least 2 y | Loss of MMR | 27 mo | 59% at 24 mo |
| Stop 2G-TKI55 | Dasatinib/nilotinib (first or second line) | 60 | MR5 for at least 24 mo | Loss of MMR | 47 mo | 54% at 48 mo |
| DASFREE56 | Dasatinib (first or second line) | 84 | MR4.5 for 12 mo | Loss of MMR | 2 y | 46% at 24 mo |
| ENESTFreedom60 | Nilotinib (first line) | 190 | MR4.5 for 12 mo | Loss of MMR | 96 wk | 49% at 96 wk |
| ENESTop study61 | Nilotinib (second line) | 126 | MR5 for at least 2 y | Loss of MMR | 96 wk | 53% at 96 wk |
| DADI57 | Dasatinib (first line) | 68 | MR4.5 for at least 24 mo | Loss of MMR | 23 mo | 55% at 6 mo |
| DADI58 | Dasatinib (second line) | 63 | MR4 for at least 12 mo | Loss of MR4 | 44 mo | 44% at 36 mo |
| EURO-SKI59 | Any TKI | 758 | MR4 for at least 1 y | Loss of MMR | 27 mo | 50% at 24 mo |
The NCCN42 and ELN44 are fairly closely aligned in their recommendations for stopping therapy. The NCCN suggests that therapy can be stopped after a patient has been in an MR4 (PCR <0.01%IS) for a minimum of 2 years and no distinction is made between stopping after first- or second-line therapy.42 The ELN recommends that therapy not be stopped if a patient has had to change therapy for lack of response, and suggests that for patients who achieve an MR,4 that therapy could be stopped after a minimum of 3 years. For patients who achieve and maintain a deeper response, MR4.5 (PCR <0.0032%), therapy could stop after a minimum of 2 years. Two factors appear to influence the likelihood of achieving a deep MR, defined as either an MR4 or MR4.5: the use of a second-generation TKI and a low-risk score at diagnosis using either the Sokal62 or EUTOS63 risk model.42 Both sets of guidelines suggest monitoring the PCR closely after cessation because most recurrences occur within the first 6 to 12 months after stopping therapy. The NCCN guidelines suggest PCR monthly for the first 6 months, then bimonthly for months 7 through 12, and then quarterly thereafter. Prompt resumption of TKI therapy should occur if MMR is lost in most instances.
This patient developed TKI withdrawal syndrome, which is typically characterized by upper body musculoskeletal pain, and usually develops within a few months of stopping therapy.54,64 This is seen in about 25% of patients who stop therapy, and is seen with all TKIs. Length of time on TKI therapy and a history of osteoarthritic-type pain have been identified as risk factors. Successful therapy includes analgesics, nonsteroidal medications, and prednisone. Another option would have been to restart TKI therapy at the original dose with a slow taper off. This particular patient’s discomfort did not respond to analgesics or nonsteroidal therapy, but resolved after a 7-day course of prednisone 10 mg daily. Based on data from both the NCCN and ELN, we have continued to observe him off therapy. His most recent PCR from October 2020 was 0.001%IS.
Final thoughts
The history of CML and its therapeutic developments represent one of the true triumphs in oncology. Most patients with this disease can usually achieve excellent control by taking 1 or 2 pills daily, and a significant proportion of these patients may even be able to stop therapy if specific therapeutic milestones are reached. Despite these achievements, treatment of CML continues to evolve, and since the last review of this topic, clinical research has brought forth new drugs (bosutinib, asciminib) and new ideas on how to use an established drug (ponatinib). Moreover, 2 other potential options, family planning and the potential for stopping therapy in certain settings, can be discussed on a patient’s initial visit. We remain indebted to Nowell, Hungerford, and Rowley for their initial observations and to all their successors from around the world who have worked and continue to work in this field.
Authorship
Conflict-of-interest disclosure: The author has served as a consultant for Novartis, Bristol Myers Squibb, and Takeda Oncology.
Correspondence: Ellin Berman, Memorial Sloan-Kettering Cancer Center, 530 East 74th St, New York, NY 10021; e-mail: bermane@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal