Abstract
Microvascular thrombosis in patients with thrombotic thrombocytopenic purpura (TTP) is initiated by GPIbα-mediated platelet binding to von Willebrand factor (VWF). Binding of VWF to GPIbα causes activation of the platelet surface integrin αIIbβ3. However, the mechanism of GPIbα-initiated activation of αIIbβ3 and its clinical importance for microvascular thrombosis remain elusive. Deletion of platelet C-type lectin-like receptor 2 (CLEC-2) did not prevent VWF binding to platelets but specifically inhibited platelet aggregation induced by VWF binding in mice. Deletion of platelet CLEC-2 also inhibited αIIbβ3 activation induced by the binding of VWF to GPIbα. Using a mouse model of TTP, which was created by infusion of anti-mouse ADAMTS13 monoclonal antibodies followed by infusion of VWF, we found that deletion of platelet CLEC-2 decreased pulmonary arterial thrombosis and the severity of thrombocytopenia. Importantly, prophylactic oral administration of aspirin, an inhibitor of platelet activation, and therapeutic treatment of the TTP mice with eptifibatide, an integrin αIIbβ3 antagonist, reduced pulmonary arterial thrombosis in the TTP mouse model. Our observations demonstrate that GPIbα-mediated activation of integrin αIIbβ3 plays an important role in the formation of thrombosis in TTP. These observations suggest that prevention of platelet activation with aspirin may reduce the risk for thrombosis in patients with TTP.
Key Points
Platelet CLEC-2 regulates VWF and GPIbα-mediated integrin αIIbβ3 activation that contributes to thrombosis in a mouse model of TTP.
Eptifibatide and aspirin decrease thrombosis in mice with TTP, documenting the clinical importance of integrin αIIbβ3 activation.
Introduction
Thrombotic thrombocytopenia purpura (TTP) is a potentially fatal disorder characterized by systemic microvascular thrombosis. Thrombosis in patients with TTP is caused by platelet adhesion to the ultra-large von Willebrand factor (VWF) multimers that result from a deficiency of ADAMTS13.1 Current treatment of acute episodes of immune TTP (iTTP) is effective.2-4 Therapeutic plasma exchange removes anti-ADAMTS13 autoantibodies and replaces ADAMTS13. Immunomodulation with corticosteroids and rituximab suppresses anti-ADAMTS13 autoantibody production. Caplacizumab, a nanobody to the A1 domain of VWF, blocks platelet adhesion to VWF, resulting in a rapid recovery of the platelet count and decreased microvascular thrombosis.5-8 However, management of patients with iTTP in remission is less clear. After recovery from an acute episode, patients may have persistent low levels of ADAMTS13 that are associated with increased risk for transient cerebral ischemic attacks9 and stroke.10,11 There is currently no effective long-term prophylactic management to decrease risk for thrombosis during clinical remission. Patients with hereditary TTP (hTTP) are at risk for thrombosis throughout their lives.12 However, only plasma prophylaxis or a “watch and wait approach” is currently recommended.3
Recent success with caplacizumab highlights the importance of VWF binding to platelet glycoprotein Ibα (GPIbα) as a key step in pathogenesis of TTP. Interaction of VWF with platelet GPIbα is critical for platelet adhesion when the vascular wall is injured.13-15 Upon VWF binding, GPIbα also transduces signals, leading to platelet activation, including activation of integrin αIIbβ3, which is important for arterial thrombosis.16,17 However, the molecular mechanism by which the GPIbα signaling is initiated and the clinical importance of GPIbα signaling-mediated integrin αIIbβ3 activation in TTP remain elusive.
In this study, we documented that GPIbα signaling-mediated integrin αIIbβ3 activation upon VWF binding required platelet C-type lectin-like receptor 2 (CLEC-2), which is primarily expressed on platelets and contributes to arterial thrombosis through a previously unknown mechanism.18,19 We found that CLEC-2 directly interacts with the extracellular domain of GPIbα in a sialylation-dependent manner, which is essential for GPIbα signaling. Deletion of platelet CLEC-2 decreased arterial thrombus formation and the severity of thrombocytopenia in in a mouse model of TTP. We further documented that treatment with eptifibatide, an inhibitor of integrin αIIbβ3 function,20 reduced thrombus formation in mice with TTP. We also observed that prophylactic oral administration of aspirin, an inhibitor of platelet activation, reduced thrombus formation in mice with TTP. Thus, our findings reveal a novel role of CLEC-2–mediated GPIbα signaling and the clinical importance of the GPIbα signaling-mediated αIIbβ3 activation in thrombus formation in TTP.
Materials and methods
Mice
Clec-2 floxed mice (Clec-2f/f) were generated as previously described.21 Platelet Clec-2–deficient mice (Plt Clec2−/−) were generated by crossing Clec-2f/f mice with a mouse line expressing Cre specifically in megakaryocytes/platelets (Pf4Cre, the Jackson Laboratory). Eight 16-week-old mice of both sexes were used. All mice were in C57BL/6J genetic background. C57BL/6J wild-type mice were purchased from the Jackson Laboratory. Experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation as well as the University of North Carolina at Chapel Hill.
TTP mouse model
The TTP mouse model was generated as previously described.22,23 Deficiency of ADAMTS13 was obtained by injecting both anti-ADAMTS13 monoclonal antibodies (mAbs) 1 3B4 and 14H7 (both 1.25 mg/kg) into mice at day 0; the negative control was obtained by injecting the nonfunctional blocking anti-ADAMTS13 mAb 20A10 (2.50 mg/kg) into mice. After 24 hours, all mice were injected with VWF (5 mg/kg) purified from human plasma via the tail vein to initiate the onset of TTP. Blood was collected 7 days before injection of VWF (day −7, baseline) and 1 day after injection of VWF for platelet counts (Hemavet), lactate dehydrogenase (LDH) activity (Gentaur, Biovision, San Jose, CA), and analysis of the multimeric pattern of VWF. After blood collection on day 1, mice were euthanized and organs were collected for histology and immunostaining analysis.
Statistics
Randomization and blinding were performed during experiments. Data were expressed as mean ± standard deviation from at least 3 independent experiments unless otherwise indicated. The two-tailed unpaired Student's t test was performed to analyze the significance by using GraphPad Prism 5, and P < .05 was considered statistically significant.
More details of materials and methods are provided in the supplemental data, available on the Blood Web site.
Results
VWF and GPIbα-mediated platelet αIIbβ3 activation requires CLEC-2
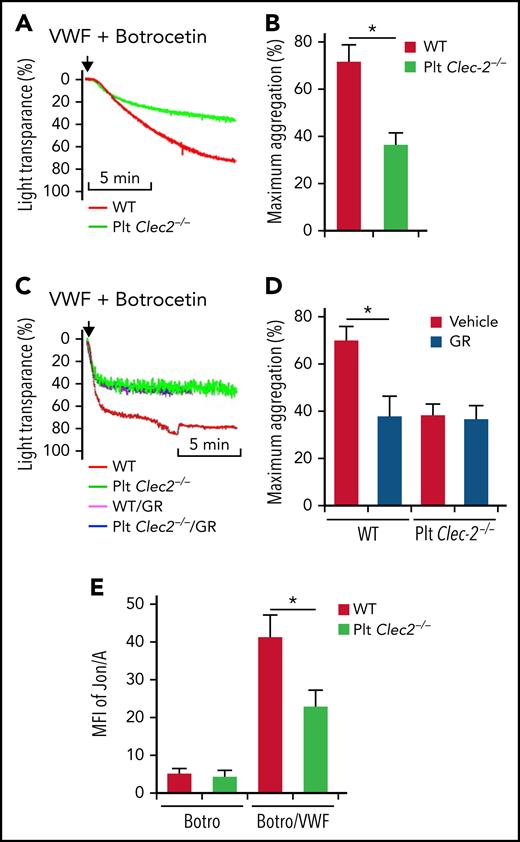
VWF binding to GPIbα initiates signaling that activates integrin αIIbβ3 in platelets.16 However, the underlying mechanism is unclear. We found that platelets lacking CLEC-2 had decreased aggregation in response to VWF (Figure 1A-B). CLEC-2–deficient and wild-type (WT) platelets had comparable aggregation in response to adenosine 5′-diphosphate (ADP) and collagen (data not shown). The surface expression of the major platelet receptors involved in aggregation, such as GPIIb, GPIbα, GPIbβ, and GPIa, were normal on CLEC-2–deficient platelets (supplemental Figure 1A-D, available on the Blood Web site), suggesting that the impaired function of GPIbα in CLEC-2–deficient platelets is not caused by altered surface expression of these receptors. Binding of VWF to WT platelets and CLEC-2–deficient platelets was similar (supplemental Figure 2A). There were no direct interactions between VWF and CLEC-2 (supplemental Figure 2B).
CLEC-2 deficiency impairs GPIbα-mediated integrin αIIbβ3 activation. Aggregation of washed WT and CLEC-2–deficient platelets in response to 10 μg/mL of VWF and 2 μg/mL of botrocetin, which facilitates VWF binding to GPIbα, in the absence (A) or presence (C) of GR144053 trihydrochloride (GR). (B and D) Quantification of platelet aggregation shown in (A) and (C), respectively. (E) Flow cytometry analysis of binding of mAb Jon/A, which specifically binds to activated integrin αIIbβ3, to platelets. Washed platelets were stimulated with 2 μg/mL of botrocetin (Botro) in the presence or absence of 10 μg/mL of VWF from human plasma. After that, platelets were immediately incubated with mAb Jon/A, and mAb Jon/A binding was analyzed with flow cytometry. The data are representative of 5 independent experiments, and data represent mean ± standard deviation (SD). *P < .05.
CLEC-2 deficiency impairs GPIbα-mediated integrin αIIbβ3 activation. Aggregation of washed WT and CLEC-2–deficient platelets in response to 10 μg/mL of VWF and 2 μg/mL of botrocetin, which facilitates VWF binding to GPIbα, in the absence (A) or presence (C) of GR144053 trihydrochloride (GR). (B and D) Quantification of platelet aggregation shown in (A) and (C), respectively. (E) Flow cytometry analysis of binding of mAb Jon/A, which specifically binds to activated integrin αIIbβ3, to platelets. Washed platelets were stimulated with 2 μg/mL of botrocetin (Botro) in the presence or absence of 10 μg/mL of VWF from human plasma. After that, platelets were immediately incubated with mAb Jon/A, and mAb Jon/A binding was analyzed with flow cytometry. The data are representative of 5 independent experiments, and data represent mean ± standard deviation (SD). *P < .05.
We then studied the role of CLEC-2 in the GPIbα-mediated αIIbβ3 activation. VWF binding to platelets is followed by GPIbα-induced integrin αIIbβ3 activation and platelet aggregation. Blocking integrin αIIbβ3 by an antagonist, GR144053, reduced VWF-induced aggregation of WT platelets but did not affect the response of CLEC-2–deficient platelets (Figure 1C-D). Binding of VWF increased recognition of WT platelets by Jon/A, an antibody that specifically recognizes activated integrin αIIbβ324 (Figure 1E). In contrast, VWF-induced binding of Jon/A to CLEC-2–deficient platelets was significantly reduced (Figure 1E).
We further tested the role of CLEC-2 in GPIbα-mediated platelet adhesion to VWF under high shear with an in vitro flow chamber system. Both WT and CLEC-2–deficient platelets adhered to immobilized VWF, but WT platelets formed much larger aggregates (Figure 2A-B). Integrin αIIbβ3 antagonist, a tripeptide Arg-Gly-Asp (RGD peptide), reduced the size of aggregates of WT platelets but not of CLEC-2–deficient platelets (Figure 2A-B). These data indicate that CLEC-2 is required for the GPIbα-mediated activation of integrin αIIbβ3.
CLEC-2 deficiency impairs platelet aggregation on immobilized VWF under flow. Washed platelets treated with albumin or 20 μg/mL of RGD, an integrin αIIbβ3 antagonist, were perfused over immobilized plasma VWF (150 μg/mL) at 10 dyne/cm2 for 10 minutes. After removing nonadherent platelets, adhered platelets were fixed with 2% paraformaldehyde (PFA), stained with anti-CD41 antibodies, and observed with microscopy. (A) Representative images of platelet aggregation on immobilized VWF under flow. (B) Quantification of platelet aggregation on immobilized VWF under flow. Adherent platelets described in (A) were quantified by analyzing the pixels of CD41-positive areas in each field. The data are representative of 5 independent experiments, and data represent mean ± SD. *P < .05.
CLEC-2 deficiency impairs platelet aggregation on immobilized VWF under flow. Washed platelets treated with albumin or 20 μg/mL of RGD, an integrin αIIbβ3 antagonist, were perfused over immobilized plasma VWF (150 μg/mL) at 10 dyne/cm2 for 10 minutes. After removing nonadherent platelets, adhered platelets were fixed with 2% paraformaldehyde (PFA), stained with anti-CD41 antibodies, and observed with microscopy. (A) Representative images of platelet aggregation on immobilized VWF under flow. (B) Quantification of platelet aggregation on immobilized VWF under flow. Adherent platelets described in (A) were quantified by analyzing the pixels of CD41-positive areas in each field. The data are representative of 5 independent experiments, and data represent mean ± SD. *P < .05.
CLEC-2 is required for GPIbα signaling
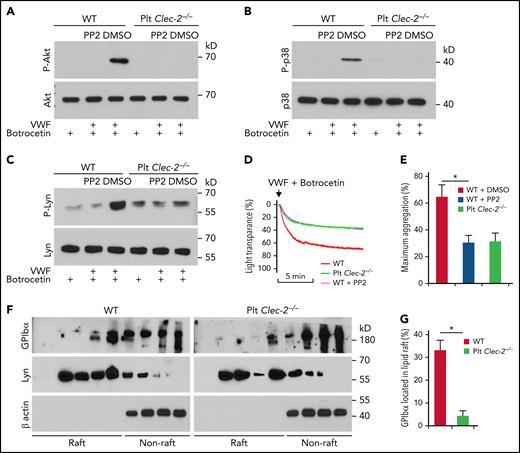
To determine how platelet CLEC-2 contributes to the GPIbα-mediated αIIbβ3 activation, we studied the Src family kinase,25Akt, and p38MAPK, which are known to be essential in GPIbα-mediated αIIbβ3 activation.25,26 VWF binding induced phosphorylation of serine on Akt and tyrosine on p38MAPK in WT platelets, respectively (Figure 3A-B). Src kinases are upstream of Akt and p38MAPK in the GPIbα signaling.25 We found that PP2, a pan-inhibitor of Src kinases, abolished VWF-induced activation of Akt and p38MAPK in WT platelets (Figure 3A-B). However, VWF binding did not induce serine phosphorylation of Akt and tyrosine phosphorylation of p38MAPK in CLEC-2–deficient platelets (Figure 3A-B). In addition, VWF binding induced tyrosine phosphorylation of Lyn, a primary Src kinase in GPIbα signaling,25 in WT but not in CLEC-2–deficient platelets (Figure 3C). Consistent with the role of Src kinase activation in GPIbα signaling, PP2 reduced VWF-induced aggregation of WT but not CLEC-2–deficient platelets (Figure 3D-E). Furthermore, we found that GPIbα in WT platelets was enriched in lipid rafts, micromembrane domains considered critical for GPIbα signaling.27 However, CLEC-2 deletion reduced the portion of raft-located GPIbα (Figure 3F-G). As controls, the distributions of Lyn, which locates in lipid rafts,28 and β actin, which is not located in cytoplasmic membrane, were not altered in CLEC-2–deficient platelets (Figure 3F-G), supporting the specificity of CLEC-2 deficiency-induced GPIbα reduction in the lipid rafts.
CLEC-2 deficiency impairs GPIbα signaling. (A) Akt, (B) p38 MAPK, and (C) Lyn activation in platelets stimulated with human plasma VWF and botrocetin, which facilitates VWF binding to GPIbα. Washed platelets were preincubated with dimethyl sulfoxide (DMSO) or 10 μM of PP2, an inhibitor of Src family kinases, at room temperature for 30 minutes and then stimulated with 2 μg/mL of botrocetin with or without 10 μg/mL of VWF for 5 minutes. Then platelets were lysed, and activation of Akt and p38MAPK was analyzed by western blotting. P-Akt, P-p38, and P-Lyn are the activated kinases. (D) Aggregation of PP2-treated platelets in response to botrocetin and VWF. Washed platelets were pretreated with DMSO or 10 μM of PP2 at room temperature for 30 minutes, and then aggregation of WT and CLEC-2–deficient platelets in response to botrocetin and VWF was observed. (E) Quantification of platelet aggregation shown in (D). (F) Flotation assay of lipid raft localization of GPIbα. Washed platelets were lysed with 1% triton X-100, and the lysate was applied to the top of 5% to 40% gradient of Optiprep. After centrifugation, the distribution of GPIbα, Lyn, and β actin in fractions from top to bottom of the gradient was analyzed by western blot. (G) Quantification of GPIbα that located in lipid rafts shown in (F). The data are representative of 5 independent experiments.
CLEC-2 deficiency impairs GPIbα signaling. (A) Akt, (B) p38 MAPK, and (C) Lyn activation in platelets stimulated with human plasma VWF and botrocetin, which facilitates VWF binding to GPIbα. Washed platelets were preincubated with dimethyl sulfoxide (DMSO) or 10 μM of PP2, an inhibitor of Src family kinases, at room temperature for 30 minutes and then stimulated with 2 μg/mL of botrocetin with or without 10 μg/mL of VWF for 5 minutes. Then platelets were lysed, and activation of Akt and p38MAPK was analyzed by western blotting. P-Akt, P-p38, and P-Lyn are the activated kinases. (D) Aggregation of PP2-treated platelets in response to botrocetin and VWF. Washed platelets were pretreated with DMSO or 10 μM of PP2 at room temperature for 30 minutes, and then aggregation of WT and CLEC-2–deficient platelets in response to botrocetin and VWF was observed. (E) Quantification of platelet aggregation shown in (D). (F) Flotation assay of lipid raft localization of GPIbα. Washed platelets were lysed with 1% triton X-100, and the lysate was applied to the top of 5% to 40% gradient of Optiprep. After centrifugation, the distribution of GPIbα, Lyn, and β actin in fractions from top to bottom of the gradient was analyzed by western blot. (G) Quantification of GPIbα that located in lipid rafts shown in (F). The data are representative of 5 independent experiments.
The extracellular domain of CLEC-2 is required for GPIbα-mediated platelet activation
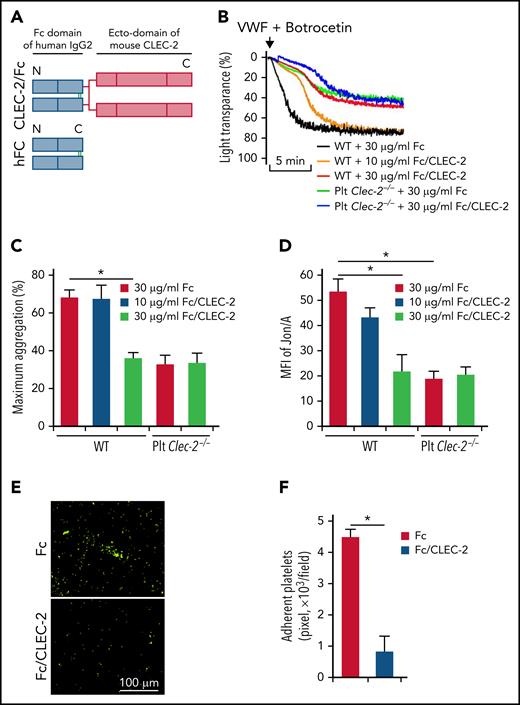
Studies have shown that the extracellular domain of CLEC-2 plays an important role in arterial thrombosis with unknown underlying mechanisms.29 We hypothesized that CLEC-2 contributes to arterial thrombosis by interacting with GPIbα through its extracellular domain. To test this hypothesis, we generated a chimeric CLEC-2 that fused the extracellular domain of mouse CLEC-2 with the Fc domain of human IgG2 (CLEC-2/Fc) (Figure 4A). Preincubation of WT platelets with the chimeric CLEC-2/Fc inhibited VWF binding-induced platelet aggregation and integrin activation under stirring or flow conditions (Figure 4B-F). Interestingly, CLEC-2/Fc did not rescue the defects in VWF-induced platelet activation of CLEC-2–deficient platelets (Figure 4B-D), suggesting that either the transmembrane domain and/or the cytoplasmic domain of CLEC-2 is also required for GPIbα signaling, which requires further studies to elucidate the complex nature of signaling transduction mediated by CLEC-2 and GPIbα.
The extracellular domain of CLEC-2 is required for GPIbα-mediated platelet activation. (A) Schematic for the construction of a chimeric CLEC-2 that fused human IgG Fc with the CLEC-2 extracellular domain (CLEC-2/FC). hFC, human IgG Fc control; N, N-terminus; C, C-terminus. (B) Recombinant CLEC-2 inhibited VWF-induced platelet aggregation. Washed WT platelets were pretreated with Fc or CLEC-2/Fc at 37°C for 1 hour, and aggregation of platelets in response of human plasma VWF was measured. (C) Quantification of platelet aggregation shown in (B). (D) Activation of integrin αIIbβ3 on CLEC-2/Fc-treated platelets. Washed WT platelets were pretreated with Fc or CLEC-2/Fc at room temperature for 1 hour and then treated with VWF in the presence of botrocetin for 5 minutes at 37°C. Then, integrin αIIbβ3 activation was measured with antibody Jon/A by flow cytometry. (E) Recombinant CLEC-2 inhibited platelet aggregation on immobilized VWF under flow. WT platelets were preincubated with Fc or CLEC-2/Fc (30 μg/mL) for 1 hour at 37°C and were then perfused over plates coated with VWF from human plasma (100 μg/mL) at 10 dyne/cm2 for 10 minutes. Adherent platelets were fixed with 2% PFA and stained with anti-mouse CD41, followed by Alexa 488-conjugated secondary antibodies. Representative images were presented. (F) Quantification of aggregated platelets shown in (E). The data are representative of 3 independent experiments and represent mean ± SD. *P < .05.
The extracellular domain of CLEC-2 is required for GPIbα-mediated platelet activation. (A) Schematic for the construction of a chimeric CLEC-2 that fused human IgG Fc with the CLEC-2 extracellular domain (CLEC-2/FC). hFC, human IgG Fc control; N, N-terminus; C, C-terminus. (B) Recombinant CLEC-2 inhibited VWF-induced platelet aggregation. Washed WT platelets were pretreated with Fc or CLEC-2/Fc at 37°C for 1 hour, and aggregation of platelets in response of human plasma VWF was measured. (C) Quantification of platelet aggregation shown in (B). (D) Activation of integrin αIIbβ3 on CLEC-2/Fc-treated platelets. Washed WT platelets were pretreated with Fc or CLEC-2/Fc at room temperature for 1 hour and then treated with VWF in the presence of botrocetin for 5 minutes at 37°C. Then, integrin αIIbβ3 activation was measured with antibody Jon/A by flow cytometry. (E) Recombinant CLEC-2 inhibited platelet aggregation on immobilized VWF under flow. WT platelets were preincubated with Fc or CLEC-2/Fc (30 μg/mL) for 1 hour at 37°C and were then perfused over plates coated with VWF from human plasma (100 μg/mL) at 10 dyne/cm2 for 10 minutes. Adherent platelets were fixed with 2% PFA and stained with anti-mouse CD41, followed by Alexa 488-conjugated secondary antibodies. Representative images were presented. (F) Quantification of aggregated platelets shown in (E). The data are representative of 3 independent experiments and represent mean ± SD. *P < .05.
Phosphorylation of the hemi-immunoreceptor tyrosine-based activation motif (hemITAM) in the cytoplasmic domain of CLEC-2 is essential for vascular integrity during inflammation.30 To address the role of CLEC-2 hemITAM in GPIbα signaling, CLEC-2 was precipitated from the lysates of WT platelets that were treated with saline, VWF, or podoplanin that activates hemITAM signaling. Western blots showed that podoplanin, but not VWF, induced tyrosine phosphorylation of CLEC-2 (supplemental Figure 3A-B). Therefore, CLEC-2 hemITAM does not contribute to VWF/GPIbα signaling.
CLEC-2 interacts with sialylated extracellular domain of GPIbα
To further address how CLEC-2 interacts with GPIbα, we conducted immunoprecipitation experiments. Chimeric CLEC-2/Fc precipitated GPIbα, but not GPVI, from WT platelet lysates (supplemental Figure 4A). Additionally, chimeric CLEC-2/Fc could not pull down GPIbα from IL4R/GPIbα mice (supplemental Figure 4A), in which the GPIbα extracellular domain was replaced by the extracellular domain of human interleukin (IL) 4 receptor.31 GPIbα has an extracellular mucin-like domain, which is heavily modified by mucin-type O-glycans capped with sialic acids (sialylated O-glycans).32 We found that removal of sialic acids from WT platelets by sialidase abolished the pull-down of GPIbα by CLEC-2/Fc (supplemental Figure 4B), indicating that sialylation of GPIbα is required to interact with CLEC-2. We further examined this interaction with a recombinant GPIbα (GPIbα-His), which fuses the extracellular domain of GPIbα with a His tag. GPIbα-His was sialylated as lectin MAL II, which recognizes α 2, 3-linked sialic acids, and detected saline-treated but not sialidase-treated GPIbα-His (supplemental Figure 4C). CLEC-2/Fc directly pulled down GPIbα-His, and this interaction was prohibited by sialidase treatment (supplemental Figure 4D). Addition of GPIbα-His to platelet lysates reduced precipitation of platelet GPIbα by CLEC-2/Fc (supplemental Figure 4E-F), confirming the sialylation-dependent interaction of GPIbα with CLEC-2.
We noticed that lack of the extracellular domain of GPIbα (IL4R/GPIbα) did not affect CLEC-2 expression or the signaling and platelet activation upon ligand binding to CLEC-2 (supplemental Figure 5A-D). Platelets from CLEC-2–deficient mice exhibited a moderate increase in their size, resembling that of platelets from IL4R/GPIbα mice (supplemental Figure 5E-F). Therefore, CLEC-2 is required for GPIbα signaling but not vice versa.
CLEC-2 regulation of GPIbα-induced platelet activation is important for thrombus formation in TTP
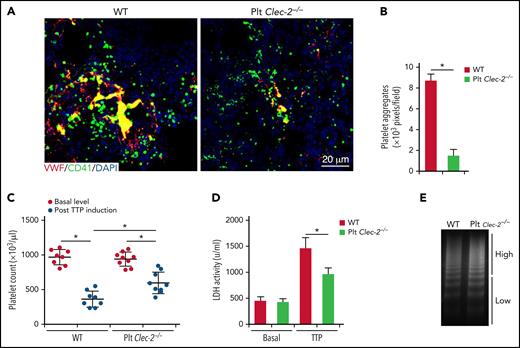
The role of GPIbα signaling in platelet activation was previously studied in vitro.25,33 However, its significance in thrombosis in vivo was not studied. Because CLEC-2 is required for GPIbα signaling, we examined thrombus development in platelet CLEC-2–deficient mice using a mouse model of TTP, in which thrombus formation is initiated by VWF binding to GPIbα. We found that thrombus formation was significantly reduced in CLEC-2–deficient mice upon induction of TTP (Figure 5A-B; supplemental Figure 6). In WT mice, VWF injection decreased the platelet count and increased plasma LDH levels, a parameter of tissue damages (Figure 5C-D). CLEC-2–deficient mice exhibited less severe thrombocytopenia and tissue damage as well as signs of thrombosis relative to that in WT mice after VWF injections (Figure 5C-D). In addition, platelet CLEC-2 deletion did not change the distribution of VWF multimers or the percentage of ultra-large VWF multimers (Figure 5E). Therefore, CLEC-2 deficiency in platelets reduces thrombus formation and decreases the severity of thrombocytopenia in the mouse model of TTP.
Platelet CLEC-2 deficiency protects mice from thrombotic injury in a mouse model of TTP. Mice were injected with antibodies to ADAMTS13 and human plasma VWF to induce TTP. At 24 hours after TTP induction, blood was collected to analyze VWF levels and platelet count, and lungs were collected to analyze thrombus formation. (A) Representative immunofluorescence images of thrombi in lungs of mice with TTP. (B) Quantification of thrombus formation in lung shown in (A). Quantification of the pixels of CD41-positive areas in each field of images. (C) Platelet counts in mice with TTP. Platelet counts in mice at 7 days before experiments (basal level) and at 24 hours after induction of TTP (by injecting antibodies to ADAMTS13 and VWF) were quantified. Each dot represented a datum from 1 mouse. Comparisons marked by asterisks are significantly different (P < .05). (D) Blood LDH activity of mice at basal level and at 24 hours after TTP induction. (E) Analysis of VWF in blood of mice with TTP by electrophoresis on agarose gel and by densitometric scanning. The data are representative of 5 independent experiments, and data represent mean ± SD. *P < .05.
Platelet CLEC-2 deficiency protects mice from thrombotic injury in a mouse model of TTP. Mice were injected with antibodies to ADAMTS13 and human plasma VWF to induce TTP. At 24 hours after TTP induction, blood was collected to analyze VWF levels and platelet count, and lungs were collected to analyze thrombus formation. (A) Representative immunofluorescence images of thrombi in lungs of mice with TTP. (B) Quantification of thrombus formation in lung shown in (A). Quantification of the pixels of CD41-positive areas in each field of images. (C) Platelet counts in mice with TTP. Platelet counts in mice at 7 days before experiments (basal level) and at 24 hours after induction of TTP (by injecting antibodies to ADAMTS13 and VWF) were quantified. Each dot represented a datum from 1 mouse. Comparisons marked by asterisks are significantly different (P < .05). (D) Blood LDH activity of mice at basal level and at 24 hours after TTP induction. (E) Analysis of VWF in blood of mice with TTP by electrophoresis on agarose gel and by densitometric scanning. The data are representative of 5 independent experiments, and data represent mean ± SD. *P < .05.
Blocking integrin αIIbβ3 activation reduces thrombus formation in TTP
Because CLEC-2 is required for GPIbα-mediated activation of integrin αIIbβ3, which is essential for thrombosis, we tested whether blocking integrin αIIbβ3 activation reduces thrombus development in the mouse model of TTP. At the time of VWF infusion, saline or eptifibatide, an integrin αIIbβ3 antagonist, was injected intravenously every 6 hours, and 24 hours later, mice were euthanized for analysis. In saline-treated mice, formation of VWF- and platelet-rich thrombi in lungs and reduced platelet counts were observed (Figure 6A-C; supplemental Figure 7). In eptifibatide-treated mice with TTP, thrombus formation was reduced (Figure 6A-B; supplemental Figure 7). Eptifibatide also decreased the severity of thrombocytopenia (Figure 6C). These findings indicate that integrin αIIbβ3 activation plays an important role in the pathogenesis of TTP.
Inhibition of integrin αIIbβ3 activation reduces thrombus formation in TTP. WT mice were injected with antibodies to ADAMTS13 and human plasma VWF to induce TTP. At 24 hours after TTP induction, blood was collected to analyze platelet count, and lungs were collected to analyze thrombus formation. Saline control or eptifibatide (15 mg/kg, body weight) was injected when VWF was administrated and then was given every 6 hours after the first administration. (A) Representative immunofluorescence images of thrombi in lungs of saline- and eptifibatide-treated mice with TTP. (B) Quantification of thrombus formation in lung shown in (A). Quantification of the pixels of CD41-positive areas in each field of images. (C) Platelet counts in mice with TTP. Platelet counts in mice at 7 days before experiments (basal level) and at 24 hours after induction of TTP were quantified. Each dot represented a datum from 1 mouse. The data are representative of 5 independent experiments, and data represent mean ± SD. *P < .05.
Inhibition of integrin αIIbβ3 activation reduces thrombus formation in TTP. WT mice were injected with antibodies to ADAMTS13 and human plasma VWF to induce TTP. At 24 hours after TTP induction, blood was collected to analyze platelet count, and lungs were collected to analyze thrombus formation. Saline control or eptifibatide (15 mg/kg, body weight) was injected when VWF was administrated and then was given every 6 hours after the first administration. (A) Representative immunofluorescence images of thrombi in lungs of saline- and eptifibatide-treated mice with TTP. (B) Quantification of thrombus formation in lung shown in (A). Quantification of the pixels of CD41-positive areas in each field of images. (C) Platelet counts in mice with TTP. Platelet counts in mice at 7 days before experiments (basal level) and at 24 hours after induction of TTP were quantified. Each dot represented a datum from 1 mouse. The data are representative of 5 independent experiments, and data represent mean ± SD. *P < .05.
Inhibition of platelet activation by aspirin reduces thrombus formation in TTP
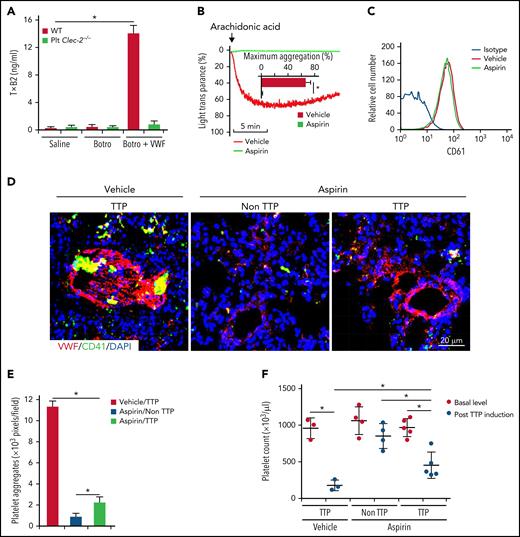
Thromboxane A2 (TxA2) is a major effector to induce platelet aggregation upon VWF binding.34 In response to VWF binding, we found that washed WT platelets released more TxA2, measured by its stable metabolite of TxB2 by enzyme-linked immunosorbent assay. However, CLEC-2–deficient platelets had significantly reduced TxA2 release upon VWF binding (Figure 7A). Because aspirin inhibits TxA2 synthesis, we examined the effect of aspirin on thrombus formation in TTP. Aspirin was given to WT mice through oral gavage35 twice a day, beginning at 5 days before induction of TTP until mice were euthanized for analysis. Aspirin administration abolished platelet aggregation in response to arachidonic acid, indicating the efficacy of aspirin treatment (Figure 7B), whereas oral aspirin did not alter platelet expression of integrin αIIbβ3 (CD61) (Figure 7C). In aspirin-treated mice with TTP, platelets adhered to VWF but did not form large thrombi in lungs (Figure 7D-E; supplemental Figure 8). Compared with saline-treated mice with TTP, thrombocytopenia in aspirin-treated mice with TTP was less severe (Figure 7F). These data indicate that oral prophylactic administration of aspirin decreases thrombus formation in TTP.
Inhibition of platelet activation by aspirin reduces thrombus formation in a mouse model of TTP. (A) Enzyme-linked immunosorbent assay analysis of TxA2 release from platelets. Washed platelets were treated with saline or botrocetin in the presence or absence of VWF for 7 minutes at 37°C, and released TxA2 was measured by detecting TxB2, a stable metabolite of TxA2, in the supernatant. TTP in WT mice was induced by injection of antibodies to ADAMTS13 and plasma VWF. Aspirin (5 mg/kg, body weight) was given 3 days before VWF administration through oral gavage, twice a day, until the end of the experiment. In mice without TTP, VWF was not administrated after antibody injection. At 24 hours after TTP induction with VWF, mice were euthanized and platelet count in blood and thrombus formation in lung were analyzed. (B) Aggregation of platelets from mice fed with the vehicle or aspirin. Platelet-rich plasma from mice treated with the vehicle control or aspirin was stimulated with arachidonic acids (500 μg/mL), and aggregation was monitored. Quantification of aggregation was inserted inside the figure. (C) Flow cytometry analysis of surface expression of CD61 on platelets from mice treated with the vehicle or aspirin. (D) Representative immunofluorescence images of thrombi in lungs of saline- and aspirin-treated mice with TTP. (E) Quantification of thrombus formation in lung shown in (D). Quantification of the pixels of CD41-positive areas in each field of images. (F) Platelet counts in mice with TTP. Platelet counts in mice at 7 days before experiments (basal level) and at 24 hours after induction of TTP were quantified. Each dot represented a datum from 1 mouse. The data are representative of 5 independent experiments, and data represent mean ± SD. *P < .05.
Inhibition of platelet activation by aspirin reduces thrombus formation in a mouse model of TTP. (A) Enzyme-linked immunosorbent assay analysis of TxA2 release from platelets. Washed platelets were treated with saline or botrocetin in the presence or absence of VWF for 7 minutes at 37°C, and released TxA2 was measured by detecting TxB2, a stable metabolite of TxA2, in the supernatant. TTP in WT mice was induced by injection of antibodies to ADAMTS13 and plasma VWF. Aspirin (5 mg/kg, body weight) was given 3 days before VWF administration through oral gavage, twice a day, until the end of the experiment. In mice without TTP, VWF was not administrated after antibody injection. At 24 hours after TTP induction with VWF, mice were euthanized and platelet count in blood and thrombus formation in lung were analyzed. (B) Aggregation of platelets from mice fed with the vehicle or aspirin. Platelet-rich plasma from mice treated with the vehicle control or aspirin was stimulated with arachidonic acids (500 μg/mL), and aggregation was monitored. Quantification of aggregation was inserted inside the figure. (C) Flow cytometry analysis of surface expression of CD61 on platelets from mice treated with the vehicle or aspirin. (D) Representative immunofluorescence images of thrombi in lungs of saline- and aspirin-treated mice with TTP. (E) Quantification of thrombus formation in lung shown in (D). Quantification of the pixels of CD41-positive areas in each field of images. (F) Platelet counts in mice with TTP. Platelet counts in mice at 7 days before experiments (basal level) and at 24 hours after induction of TTP were quantified. Each dot represented a datum from 1 mouse. The data are representative of 5 independent experiments, and data represent mean ± SD. *P < .05.
Discussion
GPIbα is well studied as a receptor to initiate platelet adhesion to the vessel wall under arterial flow conditions by interacting with VWF.15,36 Multiple studies have shown that the role of GPIbα in arterial thrombosis is more than the initiation of platelet adhesion on exposed VWF in the injured vessel.37,38 Our data document the important role of GPIbα signaling-triggered integrin αIIbβ3 activation in arterial thrombosis in TTP.
GPIbα has a critical signaling function; however, how the GPIbα-mediated signaling is initiated upon binding to VWF has been unclear. Our results demonstrate that CLEC-2 regulates GPIbα-mediated signaling and the consequent αIIbβ3 activation. Recent studies show that the extracellular domain of CLEC-2, but not the hemITAM signaling of CLEC-2, is required for arterial thrombosis.19 However, podoplanin, the only physiological ligand for CLEC-2, is not expressed on blood cells or blood vascular endothelial cells in thrombosis models18 and therefore cannot be involved in arterial thrombosis. How CLEC-2 contributes to arterial thrombosis remains to be addressed. In this study, we discovered that CLEC-2 interacts with the extracellular domain of GPIbα in a sialylation-dependent manner, which contributes to the localization of GPIbα into lipid rafts and facilitates GPIbα signaling-mediated αIIbβ3 activation.
These observations are consistent with a previous study documenting that the snake venom aggretin, a CLEC-2 ligand, pulls down GPIbα.39 In addition, our previous study demonstrated that CLEC-2 binding to its endogenous ligand podoplanin requires sialylated O-glycans on the extracellular domain of podoplanin.40 Like podoplanin, the extracellular domain of GPIbα is also modified by mucin-type sialylated O-glycans.41,42 Removing sialylated O-glycans by enzymatic cleavage reduces VWF-induced platelet activation.43 Consistent with this, we found that sialylation of GPIbα is required to interact with CLEC-2. Interestingly, both CLEC-2 and GPIbα are expressed at similar levels on platelets, as demonstrated by a recent proteomics study.44 Of note, the copy number of CLEC-2 was previously considered much lower than that of GPIbα on platelets based on an early study using antibody binding with flow cytometry.45 However, binding of antibodies to CLEC-2 is known to induce internalization of CLEC-2.46 Thus, the copy number of CLEC-2 on platelets measured with antibody binding may be artificially much lower than its true copy number. CLEC-2–deficient mice and IL4R/GPIbα mice share some phenotypes, including increased size of platelets and moderately increased tail bleeding time,31 which are reminiscent, albeit less severe, of phenotypes of Bernard-Soulier syndrome. Therefore, our data not only show that CLEC-2 physically interacts with GPIbα but also suggest that CLEC-2 may be a part of the GPIb-IX-V complex, at least in some conditions such as upon VWF binding.
We found that CLEC-2 deficiency results in GPIbα reduction in the lipid rafts in platelets, supporting a potential mechanism by which CLEC-2 regulates GPIbα signaling. Location of GPIbα in lipid rafts has been reported for 20 years with unclear mechanisms.27 CLEC-2 should not alter the integrity and composition of lipid rafts because CLEC-2–deficient platelets respond normally to ADP and collagen, which is dependent on lipid rafts.47-50 It has been reported that both N- and O-glycans enhance membrane receptors to locate into lipid rafts.51,52 Our data suggest that glycan-dependent interaction between CLEC-2 and GPIbα may contribute to their localization into lipid rafts, which requires further study.
In this study, we used a mouse model of TTP following an infusion of VWF after blocking ADAMSTS13 with antibodies, which has greater relevance for the first 24 hours of acute iTTP. We chose this model because our study focused on the CLEC-2/GPIbα signaling-mediated platelet activation in the initiation of thrombus formation in TTP. This model may not represent the entire course of a TTP episode because subsequent thrombolysis without additional supplementation of VWF may reduce thrombosis after 24 hours. The clinical importance of these observations is supported by the role of GPIbα signaling αIIbβ3 activation in thrombosis in this acute TTP model. In addition, deletion of platelet CLEC-2 impaired GPIbα signaling-induced activation of integrin αIIbβ3 and decreased thrombus formation in our mouse model of TTP. We also documented that blockage of integrin αIIbβ3 by eptifibatide or inhibition of platelet activation by aspirin reduced thrombus formation and the severity of thrombocytopenia with a mouse model of TTP.
The role of platelet activation in the pathogenesis of TTP has not been well studied. Our data show that activation of integrin αIIbβ3 is important for thrombus formation in a mouse model of TTP. Our data also show that CLEC-2 specifically controls the GPIbα signaling-induced activation of integrin αIIbβ3. Deletion of platelet CLEC-2 decreased thrombosis and the severity of thrombocytopenia. These findings indicate that GPIbα signaling upon VWF binding is the primary stimulus for platelet activation during TTP.
Current management of acute episodes of TTP is effective.2,3 However, there is currently no effective long-term prophylactic management to decrease risk for thrombosis for patients with iTTP during clinical remission. Also, current prophylactic management of patients with hTTP is not effective.12 Integrin αIIbβ3 plays an essential role in the formation of arterial thrombi, as evidenced by the effectiveness of multiple antagonists of integrin αIIbβ3 as anti-thrombotic therapy.53 In early clinical practice, preceding documentation of effectiveness of therapeutic plasma exchange, prevention of platelet activation by dipyridamole and aspirin was a standard treatment for acute episodes of iTTP.54 Because of the effectiveness of plasma exchange, dipyridamole and aspirin became obsolete. Here, we have documented the importance of integrin αIIbβ3 in thrombus formation during TTP, which will provide a potential strategy for prevention of thrombosis for patients with iTTP after effective treatment of an acute episode and for patients with hTTP, perhaps for a lifetime.
Our observations suggest that GPIbα-triggered signaling is the major approach to activate integrin αIIbβ3 that contributes to thrombosis in TTP. Most importantly, we found that inhibition of platelet activation by aspirin significantly reduced the severity of TTP. Aspirin inhibits the cyclooxygenase-1 enzyme activity in platelets that reduces the production of an important platelet agonist, TxA2. Integrin αIIbβ3 is activated by the released TxA2 and ADP from platelets when VWF binds GPIbα.16,17 Therefore, aspirin may reduce thrombosis in TTP by inhibiting GPIbα-induced TxA2 release. Because aspirin is an effective treatment to reduce the risk for stroke,55,56 it may be beneficial during remission of iTTP, when risk of stroke may be increased.10,11 Aspirin may also provide effective stroke prophylaxis for patients with hTTP. The risk for increased bleeding caused by aspirin prophylaxis should be minimal, even if patients with TTP become thrombocytopenic. The safety of aspirin prophylaxis is suggested by the uncommon risk for severe bleeding caused by caplacizumab, which is used for treatment of patients with iTTP who have severe thrombocytopenia.7,8 In the 175 patients receiving caplacizumab in these 2 reports, 47 (25%) had bleeding symptoms, but only 7 (4%) were described as severe, requiring caplacizumab interruption.7,8
Our data indicate that aspirin, an inexpensive, convenient, and relatively safe medicine, may play an important role in thromboprophylaxis for both iTTP and hTTP.
Acknowledgments
The authors thank Rodger McEver for comments.
This work was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (HL149860, HL153728, HL128390, and R35HL144976), Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (HD083418), National Institute of General Medical Sciences, National Institutes of Health (GM114731 and GM139763), and the American Heart Association (AHA18CDA34120009).
Authorship
Contribution: B.S. and L.X. designed the research, analyzed data, and wrote the manuscript; B.S., C.H., H.S., Y.K., R.H.L., X.S., J.S., J.M.M., M.Z., and S.M. performed experiments and analyzed data; J.C., J.A.L., W.B., and K.V. provided key reagents, mice, and comments; and J.N.G. interpreted the data and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Lijun Xia, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 N.E. 13th St, Oklahoma City, OK 73104; e-mail: lijun-xia@omrf.org.
REFERENCES
Author notes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Using a mouse model of thrombotic thrombocytopenic purpura (TTP), Shao and colleagues show that the deletion of platelet CLEC-2 decreases pulmonary arterial thrombosis and the severity of thrombocytopenia. They also show that an integrin αaIIbβ3 antagonist, eptifibatide, or aspirin reduces pulmonary arterial thrombosis in the TTP mice. These data indicate that platelet CLEC-2 regulates GPIbα-mediated activation of integrin αIIbβ3 in TTP and may be pharmacologically manipulated for therapeutic benefit.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal