In this issue of Blood, Ludwig et al characterize a rare form of inherited hemolytic anemia. They identified novel mutations in an intrinsically disordered region (IDR) in the GATA1 carboxyl (C)-terminal domain, which underlie novel functions in selectively regulating GATA1 chromatin occupancies in erythropoiesis.1
The authors previously described 3 families with a very rare form of X-linked inherited hemolytic anemia, characterized by shortened red blood cell lifespan, altered morphology, increased reticulocyte production, bone marrow hyperplasia, and high levels of adenosine deaminase (ADA) in mature red blood cells.1 Whole-exome sequencing identified 2 novel missense mutations in the X-linked GATA1 gene that converted a highly conserved arginine at position 307 to cysteine or histidine (R307C/H; see figure). This finding explains the X-linked inheritance of the disease.
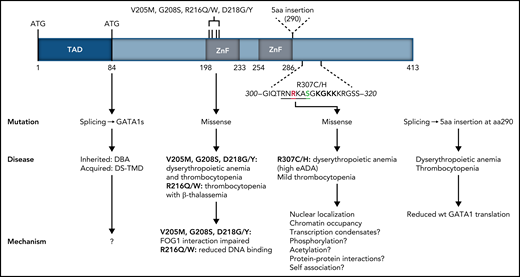
Structure of the human GATA1 protein. GATA1 mutations found in patients with congenital red blood cell disorders and thrombocytopenias are indicated, with their clinical manifestation and the possible molecular mechanisms underpinning them.1,9,10 TAD, N-terminal activation domain deleted by missense mutations resulting in expression of GATA1short associated with DBA and transient myeloproliferative disease in Down syndrome. Amino acids 300 to 320 located C-terminally to the second DNA binding ZF are shown, with R307 shown in red and phosphorylatable S310 shown in green. The AKT phosphorylation consensus sequence is underlined. The acetylatable lysine cluster is shown in bold. Amino acids 300 to 320 largely overlap the IDR described by Ludwig et al. aa, amino acids; DBA, Diamond-Blackfan anemia; DS-TMD, transient myeloproliferative disease associated with Down syndrome.
Structure of the human GATA1 protein. GATA1 mutations found in patients with congenital red blood cell disorders and thrombocytopenias are indicated, with their clinical manifestation and the possible molecular mechanisms underpinning them.1,9,10 TAD, N-terminal activation domain deleted by missense mutations resulting in expression of GATA1short associated with DBA and transient myeloproliferative disease in Down syndrome. Amino acids 300 to 320 located C-terminally to the second DNA binding ZF are shown, with R307 shown in red and phosphorylatable S310 shown in green. The AKT phosphorylation consensus sequence is underlined. The acetylatable lysine cluster is shown in bold. Amino acids 300 to 320 largely overlap the IDR described by Ludwig et al. aa, amino acids; DBA, Diamond-Blackfan anemia; DS-TMD, transient myeloproliferative disease associated with Down syndrome.
GATA1 is the master erythroid transcription factor (TF). It is a 413-amino-acid-long protein with 2 zinc finger (ZnF) domains mapping to the C-terminal half of the protein (see figure). GATA1 mutations have been linked to congenital dyserythropoietic anemia and fall into 2 main classes: (1) splicing or start codon mutations leading to expression of a shorter GATA1 protein (GATA1s) starting at amino acid 84, or (2) mutations in the first ZnF domain, disrupting interactions with cofactors (see figure). Inherited GATA1s mutations are linked to Diamond-Blackfan Anemia (DBA), whereas somatically acquired GATA1s mutations are linked to transient myeloproliferative disease in Down syndrome. The identification of the R307C/H mutations described here adds to the list of GATA1 mutations associated with disease.
The impact of the R307C mutation in erythropoiesis was investigated in detail by the authors using bone marrow mononuclear cells from a patient in an in vitro erythroid differentiation assay. Impaired erythropoiesis was evident with drastically reduced cell proliferation, loss of differentiation-associated cell surface markers, and altered cell morphology. Overexpression of either of the R307C/H mutants by lentiviral transduction in the patient’s primary cells resulted in a moderate improvement of erythropoiesis. Expression profiling of the R307C/H transduced cells showed an incomplete repression of early hematopoietic genes, including GATA2, KIT, and RUNX1, and an impaired activation of genes normally upregulated with erythroid cell maturation. Expression of the R397C/H mutants resulted in upregulated ADA expression in transduced cells. These findings were also supported by single-cell expression profiling and by similar experiments in G1E cells, a GATA1–null murine proerythroblastic cell line that serves as a model for GATA1-dependent terminal erythroid differentiation, upon the inducible restoration of virally transduced GATA1 expression.2 Together, these findings show that the R307C/H mutations impair GATA1 transcriptional activity in both human and murine erythropoiesis, while preserving some functionality that guarantees the production of red blood cells, albeit defective.
How do the R307C/H mutations impair GATA1 function? The authors noted that R307 lies in an IDR proximal to the DNA binding domain (DBD) in the second ZnF (see figure). IDRs are often found in TFs flanking DBDs; they have been implicated in modulating DNA binding specificities by the DBDs and, possibly, in the recruitment of TFs to transcriptional condensates (ie, high concentrations of transcription complexes at specific gene loci) through protein-protein interactions.3 However, protein interactome analysis of the R397C/H mutants in transduced G1E cells did not provide meaningful new insight as to IDR-mediated protein interactions that were disrupted by the mutations. Moreover, it was noted that the R307C/H mutations may be disrupting a predicted nuclear localization signal. Indeed, expression in G1E cells of R307C/H GATA1 results in an ∼40% reduction in nuclear localization, compared with wild-type (wt) GATA1. Using increasing amounts of β-estradiol to titrate nuclear levels of a GATA1-estrogen receptor fusion protein in G1E cells, showed that genes that were downregulated by R307C/H responded positively to increasing nuclear GATA1. These findings support the notion that the effects of the R307C/H mutations are, at least in part, due to reduced nuclear GATA1 levels and provide further evidence as to the sensitivity of erythroid differentiation to GATA1 dosage.4 However, it is not presently known whether the R397C/H mutations actually affect nuclear GATA1 levels in patients.
The authors next assessed chromatin occupancies during differentiation of primary erythroblasts from a patient harboring the R307C mutation. There was extensive overlap in R307C and wt GATA1 binding across the genome; however, differences were also evident with reduced R307C occupancies in key erythroid gene loci and increased occupancies in other genes, including ADA. Interestingly, differences in occupancies were observed even within the same gene locus, although no obvious underlying DNA features were identified that could account for these differences. Differential chromatin occupancies were further substantiated in G1E cells transduced with either the R307C/H or the wt GATA1. Overall, there was good concordance between changes in wt or mutant GATA1 occupancies and changes in chromatin accessibility and the direction of gene expression.
Taken together, this work identifies R307 as an important functional residue for GATA1 chromatin occupancies of target genes associated with late red cell maturation, which explains the hemolytic anemia associated with R307 mutations. The mechanism or mechanisms by which R307 regulates chromatin occupancies remain unclear; however, prior work provides some clues. First, NMR studies of the chicken GATA1 DNA binding ZnF showed that the highly conserved amino acid sequence around R307 (see figure) contacts the minor groove of the DNA across the GATA motif, with R307 itself making direct contact.5 This is consistent with properties ascribed to arginine residues within IDRs in proximity to DBDs.3 Second, R307 lies within a consensus AKT phosphorylation sequence (RNRKAS; see figure), which, upon erythropoietin (EPO) stimulation, becomes phosphorylated at S310, resulting in enhanced GATA1 transactivation functions.6 Third, a cluster of lysine residues to the C-terminus of R307 can be acetylated by CBP/p3007,8 and may modulate DNA binding, chromatin occupancy, transactivation, protein-protein interactions, and protein stability (Gutiérrez et al4 and references therein). The identification by Ludwig et al of the functional importance of R307 within an IDR provides a conceptual framework that may unite all prior observations on the GATA1 C-terminal domain, through IDR functions in transcriptional condensates in gene activation.3 This work also highlights how deregulation of IDR-mediated TF functions can result in disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal