Lenalidomide and anti-CD20-based combinations have validated efficacy in treatment of follicular lymphoma in both the frontline and the relapsed settings. In this issue of Blood, Bachy et al1 report the tolerability and efficacy of obinutuzumab and lenalidomide in the frontline therapy of follicular lymphoma.
Patients with follicular lymphoma display a wide spectrum of clinical presentations. While some patients are suitable for observation with low-volume advanced-stage disease, most patients will eventually require treatment.2 In just the last 10 years, frontline therapy for follicular lymphoma has evolved from anti-CD20 antibody with chemotherapy-based regimens to multiple options, including immunomodulatory treatment with therapies like lenalidomide with rituximab or obinutuzumab.3 Regimens without chemotherapy holds promise as the majority of patients with follicular lymphoma are older with a variety of comorbidities and benefit from treatments with reduced long-term toxicities.
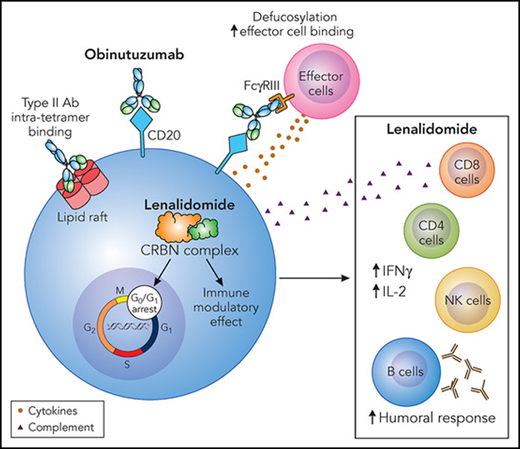
Obinutuzumab is an engineered humanized IgG1k antibody with more effective anti-lymphoma activity in both in vitro and animal models than other anti-CD20 antibodies (see figure).4 Glycoengineering reduced fucosylation in the Fc domain, which generated greater antibody dependent cellular toxicity through enhanced binding to FcγRIII receptor on immune effector cells. A modified elbow hinge binding site to CD20 facilitates a type II antibody effect by decreasing complement dependent cytotoxicity and inducing less lipid rafting and internalization of CD20.4 While clinical efficacy of obinutuzumab in diffuse large B-cell lymphoma has been disappointing, obinutuzumab with chemotherapy has demonstrable improved progression-free survival (PFS) compared with rituximab in follicular lymphoma.5
Lenalidomide is an immunomodulatory therapy that binds the cereblon E3 ligase complex and triggers ubiquination and degradation of Ikaros and Aiolos, which are key transcription factors in B and T cells (see figure).6 As a result, lenalidomide results in cell cycle arrest at G1, and reduced proliferation. Lenalidomide upregulates immune responses by increasing anti-inflammatory cytokines, enhancing immune synapse formation, and increasing T-cell effector activity.7
The Lysa group study by Bachy et al is a phase 2 clinical trial from 21 centers in France and Belgium that evaluated the combination of obinutuzumab and lenalidomide in the frontline treatment of follicular in patients meeting Groupe d’Etude des Lymphomes Folliculaires criteria. Treatment consisted of obinutuzumab and lenalidomide induction for 6 months followed by maintenance for 2 years, the first year with the obinutuzumab every 8 weeks and reduced dose lenalidomide, and a second year with only obinutuzumab every 8 weeks. The population included 43% of patients with high follicular lymphoma international prognostic index, and >30% with a bulky lymph node >7 cm. The current combination produced an overall response rate (ORR) of 92% and complete response (CR) of 47% at the end of induction, and ORR 79%, CR 63% at the end of therapy based on International Working Group 1999 criteria. Understanding the challenges and appropriateness of cross trial comparison, this represents a 15% absolute increase of patients achieving a complete remission compared with the rituximab-lenalidomide arm of the frontline RELEVANCE trial.3 The results with the obinutuzumab-lenalidomide combination using metabolic responses from Lugano 2014 criteria are more impressive with 80% achieving a metabolic complete remission at the end of induction. At nearly 4 years of follow-up, the median PFS is 82%. Another study of obinutuzumab and lenalidomide in untreated follicular lymphoma has reported similar efficacy using the Lugano 2014 criteria.8 Furthermore, the 6-year follow-up data from the frontline study of rituximab and lenalidomide compared with chemotherapy demonstrated similar PFS of 60% and overall survival of 89% in the 2 groups, suggesting that an anti-CD20 antibody and lenalidomide combination resulted in sustained clinical responses.9 Although the mechanism of action of obinutuzumab favors it as a more effective anti-CD20 antibody, it remains uncertain as to whether rituximab or obinutuzumab is the more effective anti-CD20 antibody when combined with lenalidomide in follicular lymphoma.
As with all treatments, clinicians must also balance treatment risks with benefits. The combination of obinutuzumab and lenalidomide showed grade 3 neutropenia as the primary toxicity. The neutropenia was easily managed with growth factor support. Other side effects include grade 1 to 2 diarrhea and constipation, and grade 1 to 2 infusion reactions from obinutuzumab. Ultimately, in the hands of these experienced clinicians, only 7 of 100 patients discontinued treatment because of the side effects experienced.
Improving usability of chemotherapy free regimens, such as obinutuzumab and lenalidomide therapy, will require enhancing our financial and clinical supportive care associated with this regimen. Both rituximab-lenalidomide and obinutuzumab-lenalidomide are included in the frontline treatment of follicular lymphoma in the National Cancer Care Network guidelines, yet the extent of widespread adoption is unclear.10 The out-of-pocket financial costs of lenalidomide can be challenging for many patients in the United States, and a streamlined and speedy process to access financial aid for this drug is needed. Development of supportive care practices for lenalidomide and obinutuzumab may also guide physicians as usage of these drug in follicular lymphoma becomes more standard. In this way, we can continue to deliver modern therapies to patients with follicular lymphoma regardless of their relation to major academic centers.
This study by Bachy et al represents a promising advancement for frontline follicular lymphoma treatment. Obinutuzumab has increased activity in follicular lymphoma possibly related to its mechanism of action. As future clinical trials are designed for follicular lymphoma, positron emission tomography–based measurements of disease are needed to evaluate metabolic response, which may be more relevant to clinical care. Although the efficacy and singular major toxicity of manageable neutropenia favor the use of this regimen in future combinations, guidelines for care management and cost of care remain important obstacles.
Obinutuzumab and lenalidomide mechanism of action. Obinutuzumab is a glycoengineering antibody to CD20 with a defucosylated Fc region and a modified elbow hinge binding site. These modifications increase effector cell binding and reduce lipid raft formation and hence internalization of CD20, leading to loss of antigen expression. Lenalidomide has a multitude of effects through binding to the cereblon (CRBN) complex. Transcriptional effects ultimately lead to G0/G1 cell-cycle arrest, increase apoptosis in tumor cells, and immune modulatory effects on bystander immune cells. This leads to increased humoral responses and alters the balance of pro- and anti-inflammatory cytokines by shifting toward an anti-inflammatory profile. Ab, antibody; IFNγ, interferon γ; IL-2, interleukin-2; NK, natural killer. Professional illustration by Patrick Lane, ScEYEnce Studios.
Obinutuzumab and lenalidomide mechanism of action. Obinutuzumab is a glycoengineering antibody to CD20 with a defucosylated Fc region and a modified elbow hinge binding site. These modifications increase effector cell binding and reduce lipid raft formation and hence internalization of CD20, leading to loss of antigen expression. Lenalidomide has a multitude of effects through binding to the cereblon (CRBN) complex. Transcriptional effects ultimately lead to G0/G1 cell-cycle arrest, increase apoptosis in tumor cells, and immune modulatory effects on bystander immune cells. This leads to increased humoral responses and alters the balance of pro- and anti-inflammatory cytokines by shifting toward an anti-inflammatory profile. Ab, antibody; IFNγ, interferon γ; IL-2, interleukin-2; NK, natural killer. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conflict-of-interest disclosure: The author declares research funding from Janssen, Novartis, Epizyme, Bayer, Autolus, and Roche; consulting/advisory boards for BMS/Juno/Celgene, Seattle Genetics, Kite, Karyopharm, TG Therapeutics, ADC Therapeutics, Genentech, and Incyte/Morphosys; receipt of honorarium from Dava Oncology, TouchIME, and Medscape; and stock ownership, none over $50 000, not including potential holdings in retirement mutual funds. The author owns shares of BMS, Pfizer, Viatris, Regeneron, Moderna, and Novavax as part of a stock portfolio.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal