Abstract
Introduction: For chronic lymphocytic leukemia (CLL), especially in the lymph node (LN) setting where cells receive proliferative and pro-survival signals, in-depth studies of altered metabolism and its relationship with therapeutic responses are still lacking. Venetoclax, a BCL-2 inhibitor currently in wide clinical use for CLL, has shown high efficiency yet emerging resistance is a growing clinical problem. In cell line models, induced resistance to Venetoclax was accompanied by profound metabolic changes 1. This is in accordance with our earlier findings on metabolic and apoptotic changes that CLL cells undergo within the LN environment 2. In the current study, we performed RNA sequencing and applied fluxomics with 13C 6-glucose and 13C 5-glutamine to investigate in detail the metabolic routes in LN CLL. This led to studies to manipulate glutamine metabolism in a venetoclax resistance model.
Methods: Peripheral blood (PB) samples from CLL patients were in vitro stimulated for 24 hrs by CD40 or B cell receptor (BCR), which are two potential key signals in LN. RNAseq analysis was compared with microarray data of paired PB/LN patient samples 3. For fluxomics, CLL cells were cultured for 2 hrs in medium containing either 5 mM 13C 6-glucose or 1 mM 13C 5-glutamine. Incorporation of 13C in metabolic intermediates was analyzed by LC-MS. For glutamine blockade, CLL cells were stimulated in presence of specific inhibitors of glutamine/glutamate metabolism or amino acid transporters. Cells were then treated with venetoclax, and viability was measured.
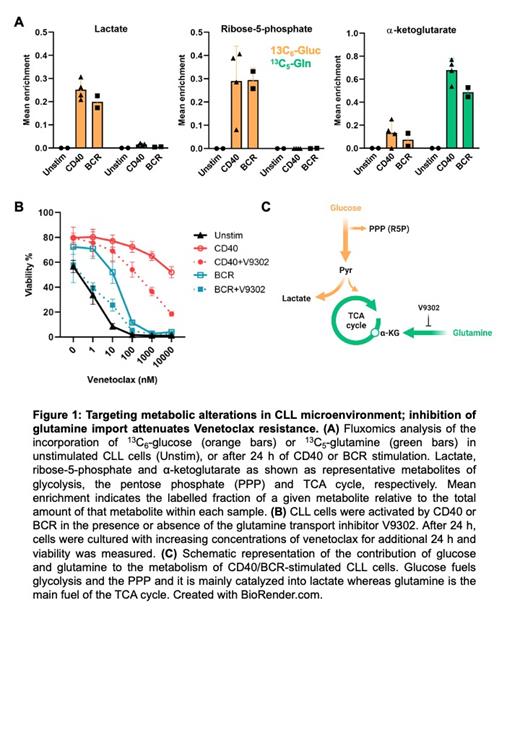
Results: Gene expression profiles demonstrated that CLL cells obtained from LN tissue as well as after in vitro CD40 or BCR stimulation showed increased expression of gene sets involved in glycolysis, oxidative phosphorylation / citric acid cycle (OXPHOS/TCA) and amino acid metabolism as well as Myc activation. This confirmed that in vitro stimulation can be used to model the CLL LN setting. For unstimulated PB CLL cells, fluxomics data demonstrated low uptake of either glucose or glutamine, with 13C labelling close to zero for most metabolites. In contrast, both CD40 or BCR stimulation increased the uptake and utilization of glucose and glutamine. 13C labelling from glucose was detected in all glycolytic intermediates analyzed in both CD40- and BCR-stimulated CLL cells. Glucose was catalyzed to lactate and also partly converted to acetyl-CoA, which entered the TCA cycle. Additionally, labelling from glucose was also increased in several metabolites of the pentose phosphate pathway (PPP) suggesting it entered nucleotide synthetic routes. Compared to glucose, the contribution of glutamine was much higher in the TCA cycle in both BCR and CD40-stimulated cells. All intermediates of the TCA cycle were highly enriched with 13C from glutamine (Figure 1A). Combined, these data revealed that glutamine is the key metabolite to fuel the TCA cycle in LN CLL cells, and prompted us to study effects of glutamine blockade in conditions of Venetoclax resistance. It was found that venetoclax resistance induced by CD40 or BCR stimulation was clearly attenuated by glutamine uptake inhibition. CLL cells became re-sensitized to Venetoclax in both CD40- or BCR-stimulated samples, with an approximate 100-fold shift in IC50 (Figure 1B).
Conclusions: Our study highlights the role of glutamine, in addition to glucose, in the metabolic reprogramming that CLL cells undergo in the LN (Figure 1C). These processes show potential for therapeutic targeting. Inhibition of glutamine import could contribute to dampen tumor microenvironment-induced Venetoclax resistance.
References
1. Guièze, R. et al. Mitochondrial Reprogramming Underlies Resistance to BCL-2 Inhibition in Lymphoid Malignancies. Cancer Cell 36, (2019).
2. Chen, Z. et al. Effects of Ibrutinib on Metabolic Alterations and Micro-Environmental Signalling in Chronic Lymphocytic Leukaemia. Blood 136, (2020).
3. Herishanu, Y. et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-κB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 117, 563-574 (2011).
Forconi: AbbVie: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Roche: Honoraria; Novartis: Honoraria; Gilead: Research Funding. van der Windt: Genmab: Current Employment. Kater: Janssen, AstraZeneca: Other: Ad Board, steering committee, Research Funding; BMS, Roche/Genentech: Other: Ad Board, , Research Funding; Abbvie: Honoraria, Other: Ad Board, Research Funding; Genmab, LAVA: Other: Ad Board, Steering Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal