Abstract
Mutations in receptor tyrosine kinases (RTK) FLT3 and KIT occur frequently in Acute Myeloid Leukemia (AML) and are associated with high risk of relapse. FLT3 tyrosine kinase inhibitors (TKI) are clinically approved in AML, but resistance is common and involves emerging clones reliant on oncogenic signaling, particularly in the RAS/MAPK pathway. Patients who relapse on FLT3 TKIs have inauspicious prognoses and no specific therapeutic options, highlighting the unmet need for effective strategies to target oncogenic signaling and improve outcomes in relapsed/refractory (R/R) AML.
The protein tyrosine phosphatase SHP2 (PTPN11) is a central node in RAS/MAPK activation downstream of various RTKs, including FLT3, acting as a scaffold for adaptor proteins that promote RAS-GTP loading. Novel allosteric inhibitors are being clinically investigated in cancers with signaling activating mutations.
Here, we demonstrate that the allosteric SHP2 inhibitor RMC-4550 modulates expression of pro and anti-apoptotics in FLT3 and KIT mutant AML providing rationale for combinatorial targeting of SHP2 and BCL2 as a synergistic approach. We subsequently report the preclinical efficacy of RMC-4550 and the FDA-approved, BCL2 selective inhibitor, Venetoclax combination in both in vitro and in vivo AML models.
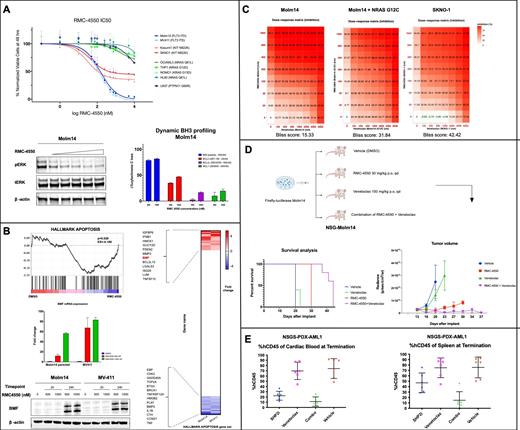
We evaluated cell viability of multiple AML cell lines treated with RMC-4550. FLT3-ITD (Molm14, MV4-11) and KIT mutant (Kasumi1, SKNO1) lines were sensitive to SHP2 inhibition. RMC-4550 maintained its efficacy in FLT3-ITD Molm14 cells with secondary mutations in FLT3 tyrosine kinase domain (TKD) and in NRAS G12C. RMC-4550 biochemically represses pERK (Figure 1A) and transcriptionally downregulates mRNA expression of DUSP6 and anti-apoptotic BCL2 and MCL1.
We functionally evaluated the mitochondrial outer membrane permeabilization (MOMP) in response to SHP2 inhibition using a dynamic iBH3 profiling assay. RMC-4550 increased the overall priming and the dependency on BCL2 in both Molm14 and MV4-11 cell lines (Figure 1A).
To investigate the global transcriptomic changes induced by allosteric SHP2 inhibition, we performed total mRNA sequencing on Molm14, MV4-11 and SKNO1 cell lines. GSEA analysis revealed that RMC-4550 significantly upregulated expression of genes repressed by RAS activation, downregulated MYC targets, but also dysregulated genes mediating apoptosis. The most consistently upregulated pro-apoptotic gene was BMF (fold change: 4.39, FDR<0.001). BMF is a BH3-only protein found to be sequestered to motor filaments that, in response to cellular damage signals, is translocated in the cytoplasm and binds pro-survival Bcl2 proteins. The BMF transcript upregulation was confirmed by qPCR and western blot analysis showed a marked overexpression of the BMF protein level upon SHP2 inhibition, particularly in the cytoplasmic subcellular compartment (Figure 1B).
We next treated Molm14, MV4-11, Kasumi and SKNO1 lines with incremental doses of RMC-4550 and Venetoclax in an 8x8 combination matrix to assess the synergy of the two compounds using cell viability and apoptosis readouts. The assay showed highly synergistic activity in both FLT3-ITD and KIT lines. Remarkably, we noted a potent synergy in Molm14 cells with concurrent mutation in NRAS G12C (Figure 1C).
In a Molm14 cell line xenograft model, we demonstrated that the combination of RMC-4550 (30 mg/kg) and Venetoclax (100 mg/kg) administered orally 5 times a week for 28 days significantly decreased leukemia burden and improved survival (p<0.001) compared to control and single agents (Figure 1D).
In a FLT3-ITD AML patient-derived xenograft (PDX) model, the combination of RMC-4550 and Venetoclax markedly decreased %hCD45 in both cardiac blood and spleen of NSGS mice compared to vehicle-treated control (Figure 1E).
Supporting a potential therapeutic index for the combination, RMC-4550 and Venetoclax strongly inhibited colony formation in FLT3 AML primary samples compared to samples from healthy volunteers.
Collectively, our data suggest that SHP2 inhibition increases the apoptotic dependency on BCL2 through up-regulation of the pro-apoptotic BMF, a mechanistic rationale to synergistically inhibit both targets. We provide preclinical evidence that co-targeting SHP2 and BCL2 is a potential effective therapeutic strategy in RTK-driven AML.
Stahlhut: Revolutions Medicine: Current Employment, Current equity holder in publicly-traded company. Smith: Daiichi Sankyo: Consultancy; Amgen: Honoraria; AbbVie: Research Funding; Revolutions Medicine: Research Funding; FUJIFILM: Research Funding; Astellas Pharma: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal