Abstract
Introduction
CD19 CAR-T is a highly effective therapy among children with relapsed/refractory B-ALL. The optimal approach to delivery of this therapy and the best post-remission strategy remain to be established. We have tested in a prospective academic trial CD19 CAR-T cells manufactured at the point-of-care based on the automatic bioreactor platform. Based on the results of the first part of the trial, a toxicity mitigation strategy with conditional split dosing and refined post-remission therapy based on allogeneic HSCT was implemented. We report here the results of toxicity mitigation strategy approach as well as the long-term outcome with regard to the HSCT consolidation.
Patients and methods
A total of 57 pts with relapsed/refractory B-ALL were screened, 54 pts were included in the trial, and additional 3 pts were eligible for compassionate use of CD19 CAR-T cell therapy. The CliniMACS Prodigy T-cell transduction process with lentiviral second generation CD19.4-1BB zeta vector (Lentigen, Miltenyi Biotec) was used for CAR-T manufacturing. All patients received prophylactic tocilizumab before CAR-T cells infusion.
In the first part of trial 33 pts received lymphodepleting chemotherapy containing cyclophosphamide (750 mg/m2) and fludarabine (120 mg/m2), CAR-T cells was administered in dose-escalating regimen (0.1, 0.5, 1, and 3х10 6/kg b.w.).
After the interim analysis, treatment scheme was modified to adapt the lymphodepletion therapy and the starting CAR-T dose to the leukemia burden. Twenty-four consecutive pts were divided into "low leukemia burden" (n=10) and "highleukemia burden" (n=14) groups, based on the threshold of 15% leukemic cells in the bone marrow.
Patients with low leukemia burden received the same lymphodepletion chemotherapy as in the first part of the trial, and a single fixed dose of CAR-T cells at 1x10 6/kg b.w.
Patients with high leukemia burden received an escalated lymphodepletion (fludarabine 120 mg/m 2, cyclophosphamide 750 mg/m 2, cytarabine 900 mg/m 2, etoposide 450 mg/m 2, dexamethasone 30 mg/m 2) and a divided dose of CAR-T. Day 0 CAR-T dose was set at 0.1 x10 6/kg. The second dose of 0.9x10 6/kg b.w. was administered between day 7 and day 14 if the following criteria were met: bone marrow leukemia burden by flow cytometry < 15% and CRS and/or ICANS grade within 3 previous days does not exceed grade 2.
Results
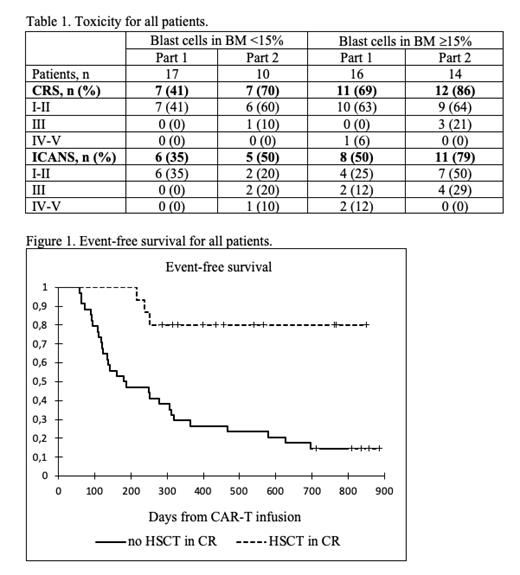
Thirty patients included in the first part of the trial, were evaluable for response at day 28, and 27 (90%) of them had MRD-negative remission. Interim analysis showed that grade 3-5 CRS and neurotoxicity were associated exclusively with large leukemia burden (>15% blasts in the bone marrow) at the enrollment (p=0,003).
With the risk-adapted strategy (part 2 of the trial), 8 patients (80%) with low leukemic burden achieved CR at day 28, and all patients (100%) with high leukemic burden achieved complete remission on day 28. In the high burden cohort 4 patients received the second CAR-T infusion, while the remaining 10 patients did not receive second dose due to either toxicity grade ³2 (4 pts), or persistence of >15% blast cells in bone marrow (6 pts). There were no cases of grade IV-V toxicity among patients with high leukemia burden, Table 1.
For all patients the median follow-up for survivors was 490 days (287-1193), the cumulative incidence of relapse after initial response was 69.6%, median time to relapse was 250 days (58-696). HSCT during the CR was performed in 15 patients. The median time between first CAR-T infusion and HSCT was 96 days (41-292). Three patients (20%) relapsed early after HSCT (88, 114 and 155 days). Event-free and overall survival for the total cohort was 19.6% and 56.4%, respectively. Among the 34 pts, who did not receive HSCT in CR after CAR-T therapy, EFS and OS were 14.7% and 55.7%. Among the 15 pts, who received HSCT as consolidation, EFS and OS were 86.1% and 80%, p-value for HSCT vs no HSCT 0.125 (OS) and 0.0001 (EFS).
Conclusion
Low doses of non-cryopreserved CAR-T cells (0.1*10 6/kg), manufactured at the point-of-care, demonstrated high efficacy in patients with high initial leukemia burden, as well as favorable profile of life-threatening toxicity. The proposed risk-adapted strategy of CAR-T dosing allows to achieve high remission rate in all patients (with high and low leukemic mass). HSCT is likely to be a necessary modality for consolidation and long-term maintenance of remission after CAR-T therapy among a majority of patients with advanced B-ALL.
Maschan: Miltenyi Biotec: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal