In this issue of Blood, Tu et al report an essential role for the autism-associated Chd8 chromatin-remodeler in the survival and genome integrity of hematopoietic stem and progenitor cells (HSPCs), through interactions with P53 and the ataxia telangiectasia mutated (ATM) protein kinase.1
Maintenance of blood cell homeostasis requires highly orchestrated changes in patterns of gene expression in stem cells and progenitors differentiating throughout the hematopoietic hierarchy. Chromatin remodeling complexes play an essential role in regulating DNA accessibility for transcription factors, other epigenetic modifiers, and the DNA repair machinery. Variations in the subunit composition of these complexes allow the coordinated expression of a large number of genes in a timely and cell-specific manner. Chromodomain helicase DNA-binding (Chd) proteins constitute a subtype of chromatin remodeling factors, which use ATP to change chromatin structure.2 Nine different Chd helicases (Chd1 to 9) have been described in vertebrates, and several studies in the last few years have unraveled their role in hematopoiesis. Of note, Chd2, 4, and 7 have been previously shown to regulate lineage differentiation, cell survival, and maintenance of genome integrity, thorough interaction with a plethora of partners, such as Runx, Ets, and Gata factors.3-5 Importantly, deficiency of CHD4 or CHD7 was shown to increase sensitization of acute myelogenous leukemia blasts to genotoxic stress and to delay leukemogenesis, respectively, making CHD helicases an attractive target for therapeutic interventions against leukemia.6,7 Now, Tu et al implicate a new Chd helicase (Chd8) in hematopoiesis and in the maintenance of HSPCs.
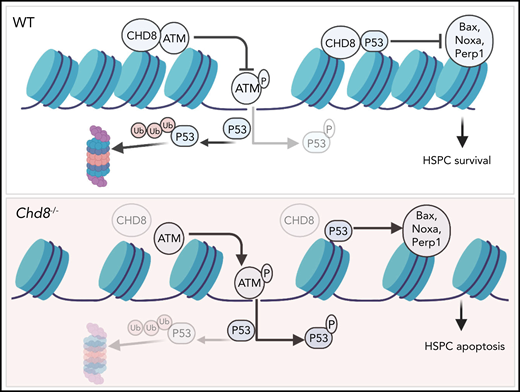
Using the Mx-Cre inducible system, Tu et al show that CHD8 deficiency results in acute pan-cytopenia and bone marrow failure in mice. Analysis of the HSPC compartment 6 days after induction of Chd8 deficiency with poly(I:C) revealed impaired stem cells reconstitution potential and a drastic depletion of hematopoietic stem cells (HSCs) and downstream multipotent progenitors as a consequence of increased apoptosis and loss of quiescence. Mechanistically, Chd8 deficiency leads to increased levels of p53 protein in HSPCs because of increased protein stability, and subsequent upregulation of p53 target genes, including Cdkn1a and the p53-induced apoptosis mediator Noxa. Chd8 was shown to form a complex with p53 and to bind directly to p53 target genes (eg, Cdkn1a). Importantly, p53 deficiency rescues the bone marrow cellularity and stem cell reconstitution defects induced by Chd8 deficiency.
Proposed model of Chd8 function in HSPCs, where it restricts p53 signaling and maintains genome integrity through interaction with the ATM protein kinase. Created with BioRender.com. See Figure 7I in the article by Tu et al that begins on page 221.
Proposed model of Chd8 function in HSPCs, where it restricts p53 signaling and maintains genome integrity through interaction with the ATM protein kinase. Created with BioRender.com. See Figure 7I in the article by Tu et al that begins on page 221.
Injection of poly(I:C) induces type 1 interferon signaling, which is a potent activator of HSCs proliferation.8,9 Interestingly, analysis of Chd8-deficient mice at later stages after poly(I:C) administration (8 weeks, as opposed to 6 days) revealed a slightly different scenario. Although these mice were still pan-cytopenic, phenotypically defined HSCs no longer showed the increased levels of apoptosis observed at earlier stages, and the numbers of immature Lin−Sca1+Kit+ progenitors were increased and showed a block in cell-cycle progression in comparison with control mice.1 Of note, similar results have also been reported by a recent study by Nita et al in Cell Reports,10 which further shows that, despite the increase in HSPCs, these were still impaired in their reconstitution capacity. The differences observed when mice are analyzed at early or later stages after poly(I:C) administration may reflect a specific requirement for CHD8 in the maintenance of HSC function in stress hematopoiesis. Importantly, Tu et al observed increased levels of the DNA damage marker γH2A.X and other features of ATM signaling pathway activation in Chd8-deficient HSPCs. These findings suggest that Chd8 plays a role in the maintenance of genome integrity, which is especially required when stem cells are recruited into active proliferation to replenish progenitors and mature blood cells. This idea is also corroborated by experiments performed by Nita et al, where reduced survival was observed when the hematopoietic system of Chd8-deficient mice was challenged with the myeloablating drug 5-Fluorouracil.10 Further studies will be necessary to elucidate the role of Chd8 in stress hematopoiesis and the physiologic relevance of the interaction between Chd8 and ATM.
CHD8 is one of the most commonly mutated genes in patients with autism spectrum disorders (ASD), which in addition to the neurodevelopmental phenotypes are also often associated with anemia and other blood disorders. The studies by Tu et al raise the possibility that mutations in CHD8 may also underly the hematopoiesis defects observed in patients with ASD. Similar to that in patients with ASD, Chd8 haploinsufficiency in mice is sufficient to develop autistic-like phenotypes. However, Chd8 haploinsufficiency in mice did not reveal major defects in hematopoiesis. Differences between mice and humans may account for this discrepancy. Alternatively, given that autism is a multifactorial disorder, additional genetic changes may be necessary to manifest the hematological phenotypes observed in patients with ASD.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal