In this issue of Blood, Silva et al demonstrate that disulfiram inhibits gasdermin D (GSDMD) and thereby inhibits the excessive formation of neutrophil extracellular traps (NETs) by neutrophilic granulocytes during sepsis.1
Neutrophils are the most prominent leukocytes in the peripheral blood, acting as a first-line defense against foreign pathogens. They are like good Samaritans, having the shortest half-life among all blood cells, sacrificing themselves while protecting the human body from invasion by bacterial, fungal, or viral infections. Neutrophils exert multiple defenses against pathogens, including phagocytosis, reactive oxygen species (ROS) production, secretion of bactericidal enzymes, and NET formation. They also pay a high price for these defensive actions, dying from the process of digesting, neutralizing, and killing foreign invaders. However, not everything is perfect in this line of pathogen defense; it is almost impossible to regulate the degree of neutrophil activation. Once activated, they often exhibit hypersensitive phenotypes, causing deleterious effects locally at the site of inflammation and systemically by playing an essential role in the initiation of multiple-organ dysfunction and the lethality of sepsis. Targeting mechanisms responsible for the deleterious effects of neutrophils during sepsis while preserving other functions may represent a valuable therapy.
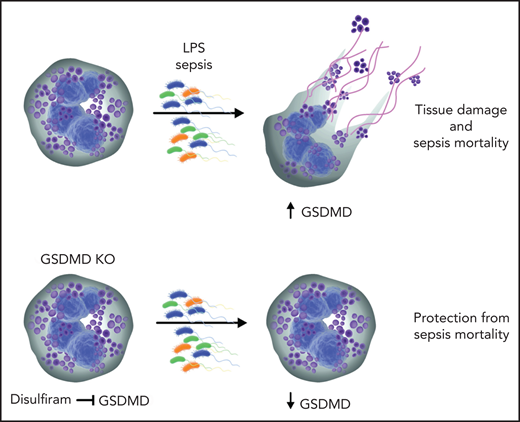
Silva et al explore the observation that excessive NET formation strongly correlates with severity of sepsis. The authors suggest that targeting NET release is a practical preventive approach to reducing organ injury during sepsis. In neutrophils, formation of NETs can be initiated in a reduced NADP (NADPH) oxidase-independent or -dependent way. The NADPH oxidase-dependent pathway starts with the generation of ROS, which in turn initiates the release and migration of neutrophil elastase (NE) in the neutrophil nucleus. The pore-forming protein GSDMD can permeabilize neutrophil granules and create a positive feedback loop of NE release and further GSDMD activation by NE.2 NE cleaves histones and assists in chromatin decondensation and release. Silva et al demonstrate that GSDMD and caspase 11 were activated in neutrophils isolated from patients and mice with sepsis. Moreover, GSDMD was detected in the typical NET structures on neutrophils from patients and mice with sepsis. The authors confirm the involvement of NE in GSDMD activation and pore formation during sepsis. GSDMD has been implicated in the pathogenesis of inflammasome-associated diseases including tissue damage during sepsis.3-5 The authors elegantly show that GSDMD deletion in mice abrogated NET formation and led to marked reduction of multiple-organ dysfunction and sepsis lethality (see figure).
Inhibition of GSDMD with disulfiram abrogates NET formation and reduces sepsis severity. During sepsis, activated neutrophils release neutrophil granule components and NETs. NETs aggravate tissue injury and may lead to death resulting from sepsis. GSDMD is proteolytically activated by neutrophil proteases during NETosis and plays a pivotal role in the generation of NETs. Disulfiram inhibits GSDMD and abrogates NET formation, thus reducing sepsis lethality. KO, knockout.
Inhibition of GSDMD with disulfiram abrogates NET formation and reduces sepsis severity. During sepsis, activated neutrophils release neutrophil granule components and NETs. NETs aggravate tissue injury and may lead to death resulting from sepsis. GSDMD is proteolytically activated by neutrophil proteases during NETosis and plays a pivotal role in the generation of NETs. Disulfiram inhibits GSDMD and abrogates NET formation, thus reducing sepsis lethality. KO, knockout.
Furthermore, the authors identify potential therapeutic compound specifically inhibiting NET formation via suppression of GSDMD. They rely on previously published data demonstrating that disulfiram is a potent inhibitor of GSDMD.6 The authors compare the effects of disulfiram treatment on NET formation by LPS-stimulated neutrophils in vitro, detecting significant reduction in NETs. In line with this, disulfiram protected mice from sepsis in the experimental in vivo model using cecal ligation and puncture and endotoxemia models. Interestingly, disulfiram is a well-known inhibitor of aldehyde dehydrogenase, which has been used for the treatment of alcoholism for many decades.7 The mechanism of GSDMD action in sepsis and its inhibition by disulfiram are not fully elucidated. There are controversial data on the different effects of GSDMD on monocytes/macrophages and neutrophils during sepsis. GSDMD induces inflammasome formation in macrophages, leading to pyroptosis, which is inflammation-induced cell death.8 In neutrophils, GSDMD amplifies neutrophil functions by potentiating NET formation. It would be interesting to investigate monocyte/macrophage functions upon treatment with disulfiram in vitro and in vivo in sepsis models. Moreover, to test the specificity of disulfiram, its effects on GSDMD−/− neutrophils need to be investigated. GSDMD−/− mice are almost completely protected from sepsis, but disulfiram only partially reduces septic lethality in these mice. A comprehensive dose-escalating analysis is warranted, and further investigation of the specificity of disulfiram for GSDMD inhibition is required. It would also be interesting to know if any residual activity of GSDMD is present in disulfiram-treated animals.
Silva et al have identified an oral drug that inhibits NET formation by blocking GSDMD activity. This finding may provide much needed help in preventing deleterious outcomes for patients with sepsis, if the laboratory results are confirmed in clinical trials. Currently, neutrophilia and excessive NET formation are adverse prognostic factors for patients with COVID-19 infection and are associated with severe cause of disease.9 Ferreira et al10 demonstrated that SARS-CoV-2 engages inflammasome and triggers pyroptosis in human monocytes, associated with GSDMD cleavage. It would be exciting to investigate whether disulfiram therapy can suppress neutrophil hyperactivation and alleviate the course of COVID-19 infection.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal