In this issue of Blood, Augsberger et al present preclinical data for a novel T-cell–bispecific antibody (TCB) that targets intracellular Wilms tumor 1 (WT1) in acute myeloid leukemia (AML). By simultaneously targeting CD3 and WT1, the authors demonstrate that WT1-TCB results in T-cell activation and killing of both AML cell lines and primary tumors in a WT1 and HLA-A2 dependent fashion with minimal effect on normal CD34+ hematopoietic cells.1
As therapeutic agents in oncology, bispecific antibodies are engineered to simultaneously bind an antigen present on a tumor cell and an immune effector cell such as T cells. In contrast to the success of blinatumomab in B-cell acute lymphoblastic leukemia, the development of bispecific antibodies in AML has been hampered by as lack of suitable target antigens. Surface antigens targeted in AML including CD33, CD123, and CLEC12A are present on normal hematopoietic stem and progenitor cells leading to prolonged cytopenias or on mature myeloid cells creating a large antigen sink that may reduce efficacy and contribute to toxicities such as cytokine release syndrome.2
For this reason, there is an expanding effort to target intracellular tumor antigens in AML and other hematologic malignancies. Intracellular tumor antigens can be degraded into peptides that are presented on the cell surface by MHC class I molecules and recognized by T cells though their T-cell receptor (TCR). Compared with antibodies, native TCRs have a lower affinity to the target antigen and often less specificity than antibodies. To overcome this limitation, TCR-like or TCR-mimic antibodies have been identified that can recognize specific peptide/MHC complexes with much higher affinities than a native TCR.
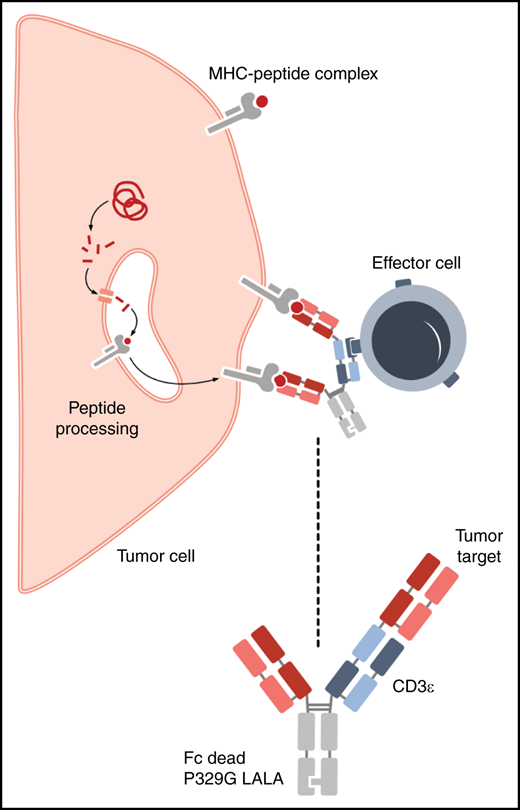
WT1-TCB is a bispecific antibody that contains a TCR-like binding domain that recognizes the WT-1 peptide, RMFPNAPYL (RMF), in the context of human leukocyte antigen-A*02 (see figure). WT-1 encodes a zinc-finger transcription factor and is an attractive immunotherapeutic target based on its overexpression in AML and a number of solid tumors with limited expression in normal hematopoietic stem cells. In AML, expression of WT-1 has been used as marker for measuring residual disease3 and has been targeted therapeutically with a variety of approaches, including monoclonal antibodies, TCR-transduced T cells, and peptide vaccines.4,5
WT1-TCB is a trivalent bispecific antibody containing 2 binding sites for a WT1 peptide presented on HLA-A*02 and a binding site recognizing CD3ε on T cells. The Fc region contains a P329G LALA mutation that increases the half-life but eliminates complement and antibody-dependent cellular cytotoxicity. See Figure 2A in the article by Augsberger et al that begins on page 2655.
WT1-TCB is a trivalent bispecific antibody containing 2 binding sites for a WT1 peptide presented on HLA-A*02 and a binding site recognizing CD3ε on T cells. The Fc region contains a P329G LALA mutation that increases the half-life but eliminates complement and antibody-dependent cellular cytotoxicity. See Figure 2A in the article by Augsberger et al that begins on page 2655.
A barrier to the clinical development of WT1-TCB is that is that the RMF peptide may not be presented on the surface of cells in a sufficient quantity to elicit a therapeutic immune response. In this paper, the authors were able to develop a highly sensitive, immunoprecipitation/mass spectroscopy assay that could detect the RMF peptide/MHC complex on the surface of primary AML cells at 152 copies per cell. As a comparison, the expression level is roughly 100-fold lower than what has been reported for surface proteins such as CD33.6
In addition, TCR-based therapies have the potential for recognition of other self-peptides leading to off-target binding and toxicity.7 A different TCR mimic antibody to WT1, ESK1, binds the same RMF peptide as WT1-TCB.8 Direct comparison of the parent antibody of WT1-TCB to ESK1 by alanine scanning shows that the 6 of 9 residues are important for binding to the RMF-MHC complex compared with ESK1 that binds primarily to N-terminal arginine residue, which may reduce off-target binding. Finally, all TCR-based therapies may be susceptible to immune escape by downregulation of either the target antigen or by MHC class I and II genes.9
Nevertheless, WT1-TCB represents a promising novel therapeutic approach in AML and forms the basis for a phase 1 clinical trial being conducted in patients with relapsed or refractory disease. (NCT04580121)
Conflict-of-interest disclosure: G.L.U. has served as a consultant for Novartis, Abbvie, Agios, GlaxoSmithKline, Jazz, and Genentech.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal