In this issue of Blood, Edwards et al report the results of a phase 1a/1b trial of passive antiamyloid immunotherapy with the monoclonal antibody CAEL-101 in patients with light chain (LC) amyloidosis with persistent organ dysfunction despite response to previous chemotherapy. Two-thirds of evaluable patients attained cardiac response and 20% reached renal response at a median time of only 3 weeks. Treatment was well tolerated. These findings open new therapeutic opportunities and create new challenges.1
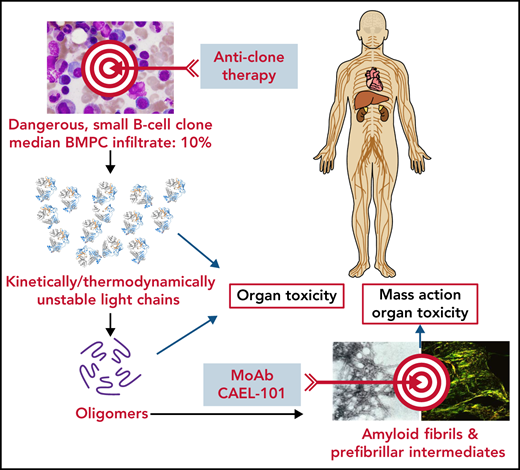
Amyloid LC (AL) amyloidosis is usually caused by a small plasma cell/B-cell clone producing an unstable immunoglobulin LC that misfolds, aggregates, and forms amyloid deposits in tissues. This process involves the heart in up to 80% of patients, and severity of cardiac damage is the main determinant of survival.2 Organ dysfunction and damage are most likely caused by toxic effects of the misfolded LCs and amyloid fibrils and disruption of tissue architecture by amyloid deposits. Therefore, in an attempt to maximize the efficacy of therapy, both ends of the amyloid cascade can be targeted (see figure).
Two-hit strategy for treatment of AL amyloidosis. Schematic representation of the amyloid cascade and targets for intervention. Suppressing the production of the amyloid precursor by anticlone immunochemotherapy extinguishes the cascade and direct organ toxicity and promotes amyloid resorption, thus favoring recovery of organ function and extending survival. Promoting amyloid resorption using passive immunotherapy should help restore tissue architecture and reduce amyloid fibril-mediated toxicity, thus improving organ function and survival. The 2-hit strategy should be synergic, abbreviating the time to organ response and possibly leading to a cure of this disease. Black arrows indicate the flow of molecular events; blue arrows indicate possible effect on target organs; red arrows indicate therapeutic interventions. BMPC, bone marrow plasma cell; mAb, monoclonal antibody.
Two-hit strategy for treatment of AL amyloidosis. Schematic representation of the amyloid cascade and targets for intervention. Suppressing the production of the amyloid precursor by anticlone immunochemotherapy extinguishes the cascade and direct organ toxicity and promotes amyloid resorption, thus favoring recovery of organ function and extending survival. Promoting amyloid resorption using passive immunotherapy should help restore tissue architecture and reduce amyloid fibril-mediated toxicity, thus improving organ function and survival. The 2-hit strategy should be synergic, abbreviating the time to organ response and possibly leading to a cure of this disease. Black arrows indicate the flow of molecular events; blue arrows indicate possible effect on target organs; red arrows indicate therapeutic interventions. BMPC, bone marrow plasma cell; mAb, monoclonal antibody.
Treatment of AL amyloidosis has been directed at reducing the concentration of the circulating precursor LCs by targeting the malignant clone with chemo- and immunotherapies.3 This strategy has been successful and has changed the face of this disease, with continuous marked improvement in survival that has now reached a median of 6 years.4 The new standard of care, targeting the plasma cell clone with daratumumab, cyclophosphamide, bortezomib, and dexamethasone, makes the amyloid LC disappear (hematologic complete response [CR]) in more than half of patients and induces cardiac and renal responses in 42% and 53% of patients, respectively.5 In AL amyloidosis, hematologic response is associated with prolonged survival, with a median >15 years in patients who achieve CR in long follow-up studies.6 Approximately 50% of patients in CR have undetectable minimal residual disease, and organ response rates exceed 90% in these patients.7
Two other antiamyloid antibodies have been tested in AL amyloidosis, but despite encouraging preliminary results,8,9 the ensuing randomized clinical trials have been interrupted for futility or unfavorable risk-benefit profile. Edwards et al targeted amyloid deposits with CAEL-101, a monoclonal antibody that reacts with a conformational epitope present on partially denatured and fibrillar LCs. Two-thirds of evaluable patients attained cardiac response, and 20% reached renal response. Cardiac response compared favorably with that reported after successful chemotherapy; however, the number of patients was small, and they were highly selected. Treatment was well tolerated, and no dose-limiting toxicity was observed.
All patients were previously exposed to 1 to 10 lines of chemotherapy, and median times from last chemotherapy administration were 2.6 and 7.4 months in the phase 1a and 1b portions of the study, respectively (range, 0-15.5 months). Twenty patients (74%) were in at least hematologic very good partial response (CR in 37% of cases) at the time of first infusion of CAEL-101, which is generally considered an adequate end point of chemotherapy to induce organ response. Therefore, although no patient had achieved organ response before CAEL-101 initiation, one cannot exclude that a late effect of successful chemotherapy, which is known to occur in this disease, may have contributed to organ response. However, the rapidity of organ response (median 3 weeks) after CAEL-101 administration suggests that treatment may have accelerated organ response, although only controlled randomized trials will answer this critical question. The authors postulate that this rapid action can be explained by the ability of CAEL-101 to reduce toxic intermediates and pathogenic amyloid deposits, thus alleviating organ damage. Indeed, rapid reduction of the concentration of N-terminal pronatriuretic peptide type B (NT-proBNP), the biomarker used to assess cardiac response in AL amyloidosis, was observed after infusion of CAEL-101 (supplemental Figure 2 in Edwards et al). The effect on cardiac function was further supported by a finding of improvement in global longitudinal strain on echocardiogram.
The capability of CAEL-101 to rapidly improve cardiac function may be particularly relevant for the 20% of patients who present with very advanced cardiac involvement, with NT-proBNP ≥8500 ng/L (stage IIIb), and who survive only a few months despite chemotherapy. This unmet need is the most challenging in the care of patients with AL amyloidosis. It is to be commended that CAEL-101 is now being tested vs placebo in 2 trials in patients with advanced (stage IIIa) and very advanced (stage IIIb) cardiac involvement receiving anticlone chemotherapy (registered at www.clinicaltrials.gov as #NCT04504825 and #NCT04512235). These trials, in addition to providing important clinical information on the impact of antiamyloid therapy on organ function and survival, will shed light on the pathogenesis of the disease. There is concern that the loss of cardiomyocytes that accompanies amyloid deposition10 may compromise recovery of cardiac activity even after amyloid reabsorption. It is hoped the trial in patients with stage IIIb disease will answer this critical question. Interestingly, although an interim analysis of the placebo-controlled phase 3 trial of another antiamyloid antibody, birtamimab, in association with chemotherapy showed no benefit and led to study termination, possible clinical efficacy was noted in patients with advanced amyloid disease. A new trial of birtamimab combined with chemotherapy is planned in these patients (registered at www.clinicaltrials.gov as #NCT04973137) and will provide valuable insights.
One may speculate that current organ response criteria validated to predict the impact of antiplasma cell therapy on survival might not be able to adequately assess the efficacy of antiamyloid therapy. The primary end point of the 3 ongoing randomized trials of passive antiamyloid immunotherapy is survival. These trials will offer the opportunity to identify new candidate organ response criteria linked to the impact of antiamyloid therapy on survival that can be used in subsequent studies.
Aiming to cure AL amyloidosis requires eliminating the supply of the AL precursor, and in all the current trials, passive antiamyloid immunotherapy is being administered in combination with chemotherapy. However, amyloid resorption triggered by antiamyloid immunotherapy requires efficient macrophage function, and possible interference of chemotherapy should be considered. However, there is hope that the ongoing trials will prove that anticlone and antiamyloid therapies can be synergistic, thus increasing the frequency and speed of organ recovery, with better quality of life and extended survival.
Conflict-of-interest disclosure: G.P. serves on an advisory board for Janssen-Cilag and has received honoraria from Janssen-Cilag. G.M. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal