In this issue of Blood, Kittai and colleagues investigate the impact of a complex karyotype on outcome in a large cohort of patients with chronic lymphocytic leukemia (CLL) treated with the BTK inhibitor ibrutinib.1 As in prior studies, they confirm that a complex karyotype, defined as ≥3 or ≥5 chromosomal alterations, is a high-risk marker in ibrutinib-treated patients. They propose using karyotypic complexity as a continuous variable for predicting outcome, as increasing numbers of aberrations correlated with decreasing survival.
By the early 1990s, Juliusson et al reported that the higher the number of chromosomal aberrations detected, the worse the outcome in patients with CLL.2 This finding was confirmed in studies applying genomic arrays, where increasing genomic complexity was associated with shorter time to first treatment and overall survival (OS).3 Because of the inherent difficulties to generate metaphase chromosomes for cytogenetic analysis in CLL, it was not until newer culturing protocols that included CpG and IL2 were introduced that most CLL samples could be karyotyped.4 In a series of studies, complex karyotype, defined as ≥3 chromosomal alterations, was found to be a high-risk factor4; also, the presence of unbalanced structural aberrations was linked to a more dismal prognosis
In a recent study published in Blood, Baliakas et al investigated the impact of a complex karyotype in more than 5200 patients with CLL.5 In this cohort, they found that for patients without TP53 aberrations, a complex karyotype was associated with high-risk disease if ≥5 chromosomal aberrations were present. On the other hand, if a patient carried a TP53 aberration [ie, del(17p) and/or TP53 mutation], the association with a worse outcome was already reached, if the patient had 3 or more alterations. Although few patients were treated with newer agents in their retrospective cohort study, the presence of a complex karyotype has been shown to be a high-risk factor in patients treated with BTK or BCL2 inhibitors, albeit mostly in smaller patient series.6
In the current study, Kittai et al explored the impact of complex karyotypes in a large, single institution cohort (n = 456), including both treatment naive (22%) and relapsed or refractory (78%) patients, treated with single-agent ibrutinib or ibrutinib in combination with an anti-CD20 monoclonal antibody. In addition to confirming the impact of a complex karyotype, defined as either ≥3 or ≥5 cytogenetic abnormalities, on outcome, they observed that patients with an increasing number of alterations, from 0, 1 to 2, 3 to 4, 5 to 9, 10 to 14, and ≥15 aberrations, had progressively worse survival curves. Hence, they assessed whether karyotypic complexity, included as a continuous variable, had an impact on outcome. Their analysis showed that accumulating karyotypic complexity was an independent variable associated with both inferior progression-free survival and OS. Furthermore, they had access to multiple cytogenetic analyses from a subset of patients progressing while on ibrutinib therapy (ie, at baseline and relapse) and demonstrate that the presence of karyotypic evolution at progression was linked to a worse outcome (see figure).
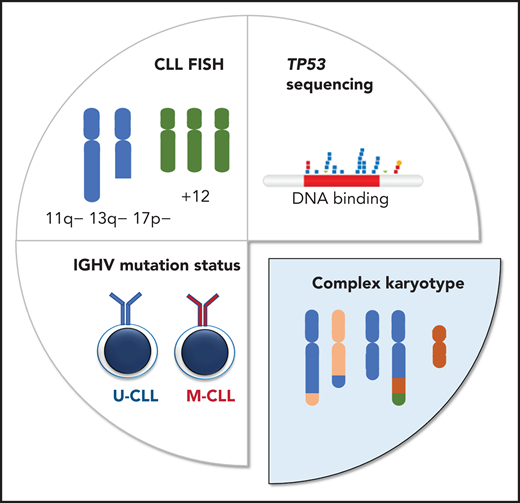
Different diagnostic methods are applied to determine clinically relevant genetic markers in CLL. Fluorescence in situ hybridization (FISH) is performed to detect the deletions of 11q, 13q, and 17p, as well as trisomy 12, whereas the IGHV and TP53 mutation status is determined by sequencing (Sanger sequencing and/or NGS). Karyotypic complexity is determined by cytogenetic analysis, counting both numerical and structural chromosomal aberrations.
Different diagnostic methods are applied to determine clinically relevant genetic markers in CLL. Fluorescence in situ hybridization (FISH) is performed to detect the deletions of 11q, 13q, and 17p, as well as trisomy 12, whereas the IGHV and TP53 mutation status is determined by sequencing (Sanger sequencing and/or NGS). Karyotypic complexity is determined by cytogenetic analysis, counting both numerical and structural chromosomal aberrations.
Today, different genetic analyses are performed to identify high-risk patients (see figure) and the present study adds further support to the clinical relevance of identifying patients with a complex karyotype. Moreover, Kittai et al show the importance of performing reanalysis at disease progression. As they rightly point out, there is a lack of consistency in the protocols used for cytogenetic analysis in CLL when the more recent culturing conditions are used. Hence, it is important to standardize protocols and even perform a multicenter evaluation to determine the reproducibility of cytogenetic analysis in CLL.
Another option would be to examine genomic arrays or perform whole-genome sequencing (WGS) to determine the degree of genomic complexity. In a recent multicenter effort using genomic arrays, the presence of ≥5 or more genomic aberrations was linked to high-risk disease.7 In fact, centers are already using genomic arrays in routine diagnostics to identify recurrent aberrations in CLL and can, at the same time, detect genomic complexity (but not translocations). The other alternative would be WGS, which has the potential, not only to determine single mutations, copy-number aberrations, and structural aberrations, but also to identify more complex markers, such as complex karyotype and IGHV gene mutation status. Although initiatives are ongoing in acute leukemia to introduce WGS instead of cytogenetic/molecular analyses, the cost of sequencing must decline further for it to be a realistic option in CLL diagnostics.
One important caveat with the current study concerns the lack of TP53 mutation status in the cohort. We know that patients with del(17p) plus TP53 mutation comprise 60% of patients with TP53 aberrations, whereas another 30% of patients have only TP53 mutations.8 In other words, a proportion of patients included in this cohort most likely carried TP53 mutations that were undetected. Considering the tight link between genomic complexity and TP53 aberrations, this information would have been valuable. In addition, we know that patients may harbor minor subclones with TP53 mutations, detected only by next-generation sequencing (NGS)-based assays, that are similarly linked to a poor response to therapy, at least to chemoimmunotherapy.9 In future studies, it is important to conclusively analyze the TP53 gene, using targeted NGS and including other genes linked to genomic instability (eg, ATM and SETD2) or with prognostic impact in CLL. In recently developed, broader NGS panels, it is also possible to include a copy-number backbone to detect larger genomic aberrations, in addition to small mutations.10
In summary, the results of Kittai et al underscore the clinical relevance of increasing karyotypic complexity in patients with CLL treated with ibrutinib. In the coming years, we should standardize protocols for cytogenetic analysis or other methods selected to identify genomic complexity and define how increasing complexity should be measured. If we decide to use complex karyotype as a continuous variable, what number of aberrations should be included for each unit increase in karyotypic complexity? Do all types of alterations have the same clinical impact? Finally, we must discuss whether NGS-based technologies could represent an alternative approach to low-resolution cytogenetics for identifying complex karyotype in CLL.
Conflict-of-interest disclosure: R.R. has received honoraria from AbbVie, AstraZeneca, Janssen, Illumina, and Roche.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal