In this issue of Blood, Greinacher et al1 propose that the pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT) involves a 2-step mechanism, initiated by binding of PF4 to components of the ChadOX1 nCov19 adenoviral vaccine followed by a prothrombotic antibody response similar to autoimmune heparin-induced thrombocytopenia.
A number of vaccines targeting the SARS-CoV-2 virus were urgently developed to curtail the infection and the complications of COVID-19. Two of these vaccines, ChAdOx1 nCoV-19 (Oxford-AstraZeneca) and Ad26.COV2.S (Johnson & Johnson/Janssen), use recombinant adenovirus vectors encoding the SARS-CoV-2 spike glycoprotein.2 These vaccines were extensively evaluated before regulatory authorization for utilization and did not demonstrate any safety concerns. However, ongoing safety surveillance identified an association between adenovirus-based vaccines and the rare development of thrombocytopenia and thrombosis in atypical locations, including the cerebral venous sinus thrombosis (CVST) and splanchnic veins 1 to 2 weeks after vaccination, termed VITT.3 Patients with VITT have a high level of circulating immunoglobulins (IgGs) that recognize platelet factor 4 (PF4) and activate platelets in a manner that shares features with autoimmune heparin-induced thrombocytopenia with thrombosis, previously unraveled by elegant work from Greinacher et al3 and Kelton and Warkentin,4 among others. In the absence of heparin exposure, the source and nature of the polyanion(s) that associate with PF4 in VITT have been heavily debated. Although VITT is rare among the millions of patients receiving adenoviral-based vaccines, a detailed understanding of the initial steps that result in VITT is needed to inform management at the bedside and the development of future vaccines and therapeutics that may use similar vectors.
To that end, Greinacher et al perform a detailed characterization of a potential mechanism underlying VITT pathogenesis using the ChAdOx1 nCoV-19 vaccine. Using three different imaging techniques, the authors demonstrate that adenovirus and vector components in the vaccine aggregate with PF4 in a charge-driven manner, to which anti-PF4 IgG binds. The authors then investigated vaccine composition and the ability of the ChAdOx1 vaccine to induce inflammation. They determined that approximately half of the proteins in the vaccine were of human origin, likely from the T-Rex HEK293 cells used in vaccine manufacturing. The investigators also identified EDTA in the vaccine. Used as an excipient, EDTA is a chelating agent that may sequester calcium necessary to maintain local endothelial barrier function. Intradermal injection of either the vaccine or EDTA alone triggered vascular leakage in mice, and reconstitution of calcium in the vaccine mitigated the loss of barrier integrity. The authors also observed that serum from patients with VITT robustly initiated platelet aggregation when presented with PF4. Notably, this effect was fully abrogated by blockade of the FcγIIa receptor (FcγRIIA). Prothrombotic neutrophil extracellular traps (NETs), known to occur in acute COVID5 and in autoimmune heparin-induced thrombocytopenia (HIT), also formed when neutrophils were stimulated with VITT serum or affinity purified anti-PF4 IgG in the presence of PF4 and platelets. Greinacher et al and another recent report6 observed that NETs were more prevalent in CVST tissue from patients with VITT compared with VITT-unrelated CVST.
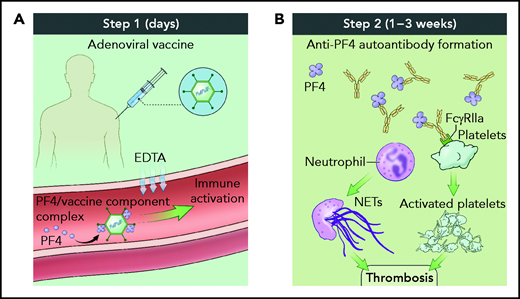
Taken together, these data support the hypothesis that VITT pathogenesis occurs in a 2-step process (see figure). In the first step, shortly after vaccine inoculation, vaccine components and PF4 form neoantigens, promoting a proinflammatory vascular milieu that amplifies the adaptive immune response including production of anti-PF4 antibodies. In the second step, 1 to 3 weeks after inoculation, complexes of polyanion/PF4/anti-PF4 antibody activate neutrophils and platelets in an FcγRIIA-dependent manner, leading to thrombosis accretion in atypical vascular beds. Because the number of patients diagnosed with VITT is low, it remains unclear whether VITT has a predilection for thrombosis in unusual sites such as the cerebral venous sinus or whether thromboses in more typical sites such as the peripheral veins do not raise the clinical alarm to trigger diagnostic testing.
Two-step process for VITT pathogenesis. (A) Shortly after vaccine administration, components of the adenovirus vaccine and PF4 generate immune complexes, whereas EDTA sequesters calcium, leading to vascular leak and an inflammatory response serving as a danger signal to provoke antibody generation. (B) One to 3 weeks after vaccine administration, polyanion/PF4/anti-PF4 antibody immune complexes trigger neutrophil extracellular trap formation and platelet aggregation in an FcγRIIA-dependent manner, resulting in thrombosis. Illustration by Alan Hoofring, National Institutes of Health.
Two-step process for VITT pathogenesis. (A) Shortly after vaccine administration, components of the adenovirus vaccine and PF4 generate immune complexes, whereas EDTA sequesters calcium, leading to vascular leak and an inflammatory response serving as a danger signal to provoke antibody generation. (B) One to 3 weeks after vaccine administration, polyanion/PF4/anti-PF4 antibody immune complexes trigger neutrophil extracellular trap formation and platelet aggregation in an FcγRIIA-dependent manner, resulting in thrombosis. Illustration by Alan Hoofring, National Institutes of Health.
These findings provide detailed insight to VITT pathogenesis and the reasons that it may occur after vaccination with adenoviral vector-based vaccines but not mRNA-based vaccines. However, many questions remain. What is the protein(s) in the vaccine that binds to PF4? What is the precise neoantigen generated when PF4 and vaccine components interact? Do human proteins in the vaccine provoke an immune response? Is the prothrombotic antibody repertoire in VITT limited to PF4, the vaccine and its components, or is there overlap with autoantibodies found in acute COVID, autoimmune disease, and other critical illnesses? What is the half-life of immune complexes or the effect of multiple vaccine doses on the autoantibody response? Ultimately, answers to these and other questions will be needed to inform future development of vaccines and therapeutics that use adenovirus- and other virus-based vectors.
As more is understood about the molecular disruptions that occur during VITT, the question remains; what is the best treatment for patients? Proposed therapeutics include IV immunoglobulin (IVIG) because of its success in treating autoimmune HIT, nonheparin anticoagulants, and plasmapheresis. A small body of evidence from nonrandomized trials and retrospective studies suggests that IVIG may be an effective treatment of VITT, although sometimes incomplete.7 The results of this study by Greinacher et al bring to light another potential therapeutic, the spleen tyrosine kinase (SYK) inhibitor, fostamatinib that is currently used for the treatment of chronic immune thrombocytopenia. Fostamatinib inhibits activation of Fc receptor by antigen/antibody complexes, and reduced NETosis and platelet activation in ex vivo COVID studies.8 In hospitalized patients with COVID, where circulating, prothrombotic antibodies that activate neutrophils, platelets, and endothelium have been identified,9 orally administered fostamatinib reduced adverse events and showed a trend toward clinical benefit.10 Although large, randomized trials are impractical in VITT given its low incidence, the FcγRIIA-dependent signaling mechanism leading to platelet activation in VITT identified by Greinacher et al provides strong rationale to consider SYK inhibition in the limited therapeutic armamentarium of clinicians treating VITT and perhaps other forms of autoantibody-mediated thrombosis.
Conflict-of-interest disclosure: Y.K. serves as a board member for the Society for Vascular Medicine, participates in the National Heart, Lung and Blood Institute (NHLBI) CONNECTS program and ACTIV-4 Host Tissue trial, and is an author on an unrelated patent application by the University of Michigan for the use of biogases in vascular disease. J.R.S. was the principal investigator of a clinical trial sponsored by the NHLBI to evaluate fostamatinib in acute COVID and participates in the ACTIV-4 Host Tissue trial.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal