Key Points
Cancer, central venous catheter, older age, and MIS-C are risk factors for thrombosis in children and adolescents with COVID-19 or MIS-C.
Mortality was high (28%) in children and adolescents with MIS-C or COVID-19 who developed thrombosis.
Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with thrombotic complications in adults, but the incidence of COVID-19-related thrombosis in children and adolescents is unclear. Most children with acute COVID-19 have mild disease, but coagulopathy has been associated with multisystem inflammatory syndrome in children (MIS-C), a postinfectious complication. We conducted a multicenter retrospective cohort study to determine the incidence of thrombosis in children hospitalized with COVID-19 or MIS-C and evaluate associated risk factors. We classified patients into 1 of 3 groups for analysis: COVID-19, MIS-C, or asymptomatic SARS-CoV-2. Among a total of 853 admissions (COVID-19, n = 426; MIS-C, n = 138; and asymptomatic SARS-CoV-2, n = 289) in 814 patients, there were 20 patients with thrombotic events (TEs; including 1 stroke). Patients with MIS-C had the highest incidence (9 [6.5%] of 138) vs COVID-19 (9 [2.1%] of 426) or asymptomatic SARS-CoV-2 (2 [0.7%] of 289). In patients with COVID-19 or MIS-C, a majority of TEs (89%) occurred in patients age ≥12 years. Patients age ≥12 years with MIS-C had the highest rate of thrombosis at 19% (9 of 48). Notably, 71% of TEs that were not present on admission occurred despite thromboprophylaxis. Multivariable analysis identified the following as significantly associated with thrombosis: age ≥12 years, cancer, presence of a central venous catheter, and MIS-C. In patients with COVID-19 or MIS-C, hospital mortality was 2.3% (13 of 564), but it was 28% (5 of 18) in patients with TEs. Our findings may help inform pediatric thromboprophylaxis strategies.
Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2019 has led to the global pandemic of a highly transmissible and severe disease called coronavirus disease2019 (COVID-19).1 Although severe pneumonia is the cardinalpresentation of COVID-19 in adults, a proclivity to cause thromboticcoagulopathy became apparent early in the pandemic.1-10 Manypatients had elevated levels of D-dimer and fibrinogen with mild thrombocytopenia and mild prolongation of prothrombin time (PT).1-3 Studies in adults reported a high venous thromboembolism (VTE) rate, often occurring despite prophylactic anticoagulation.4-9 A recent meta-analysis estimated an overall VTE incidence of 21% in adults hospitalized for COVID-19, rising to 31% in those admitted to the intensive care unit (ICU).10 The pooled odds of mortality were 74% higher among adult patients with thrombotic events (TEs), compared with those without TEs (23% vs 13%, respectively).10
Compared with adults, most children and adolescents with COVID-19 have minimal disease, and many infected with SARS-CoV-2 are asymptomatic.11 Those age <21 years accounted for only 8% of reported cases and 0.08% of deaths in the United States.12 Starting in April 2020, reports emerged of previously healthy children presenting with fever, cardiovascular shock and/or Kawasaki disease features, hyperinflammation, and multisystem involvement with a temporal association with SARS-CoV-2 exposure.13,14 Many patients were SARS-CoV-2− on respiratory testing but had positive antibody titers. Public health alerts from the Centers for Disease Control and Prevention (CDC) and the World Health Organization listed criteria for this new disease called multisystem inflammatory syndrome in children (MIS-C).15-17 Coagulopathy was listed as a potential presenting feature of MIS-C.
As hospitalizations for COVID-19 increased, pediatric hematologists developed guidelines for thromboprophylaxis.18 The Pediatric/Neonatal Scientific and Standardization Subcommittee of the International Society of Thrombosis and Haemostasis published consensus guidelines recommending pharmacologic prophylaxis in children with additional risk factors for VTE or those with elevated D-dimer.19 These guidelines were based on extrapolation of adult data and expert opinion and not supported by data on the incidence of or risk factors for thrombosis in children or adolescents with COVID-19 or MIS-C. Therefore, the primary objective of this study was to determine the incidence of thrombotic complications in hospitalized children and adolescents with COVID-19 or MIS-C. We additionally sought to evaluate risk factors associated with TEs and describe the current practices for thromboprophylaxis in this cohort.
Methods
Study cohort
We conducted a retrospective cohort study of consecutive children age 0 to <21 years admitted from 1 March 2020 through 15 August 2020 with SARS-CoV-2+ polymerase chain reaction (PCR) or diagnosis of MIS-C across 7 pediatric hospitals in 6 US states: Boston Children’s Hospital (Massachusetts), Children’s Hospital of Los Angeles (California), Children’s Hospital of Philadelphia (Pennsylvania), Children’s Medical Center of Dallas (Texas), Johns Hopkins All Children’s Hospital (Florida), Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center (New Jersey), and Texas Children’s Hospital (Texas). This study was approved for a waiver of informed consent by each center’s institutional review board.
Eligible patients were identified by ≥1 of the following: COVID-19 or MIS-C patient databases maintained at participating institutions, electronic medical record queries for patients with both an International Classification of Diseases (10th revision) COVID-19 diagnosis code and an inpatient admission code, and/or electronic medical record queries for patients with a SARS-CoV-2+ test and coded for inpatient admission. Additionally, MIS-C patients were identified from electronic medical record queries for inpatients who had a consult order with the consult reason MIS-C and/or from electronic medical record queries for patients who had a COVID-19–related laboratory test and at least 1 of the following tests sent: ferritin, interleukin-2, or interleukin-6.
The following data were collected and entered into Research Electronic Data Capture software20,21 : demographics, comorbidities, COVID-19 exposure history and presenting symptoms, hospital course (ICU admission; need for ventilator, vasopressors, or extracorporeal membrane oxygenation; presence of bacterial coinfection; presence of central venous catheter [CVC]; length of stay; and death), use of prophylactic anticoagulation (drug, dose, and duration), use of COVID-19– or MIS-C–directed therapies, hematologic and inflammatory laboratory parameters, presence of so-called COVID toes,22 presence of thrombosis up to 30 days postdischarge, and presence of bleeding during hospitalization.
Study definitions
Admissions were categorized into 1 of 4 subgroups at the discretion of the investigator: COVID-19, a positive PCR and COVID-19 symptoms (fever, cough, diarrhea, loss of smell, headache, sore throat, or congestion); MIS-C, all 5 CDC criteria of fever, evidence of inflammation on laboratory testing, ≥2 organ systems involved, no other plausible diagnosis, and SARS-CoV-2+ PCR or antibody test or known exposure23 ; MIS-C like, symptoms and laboratory findings highly suggestive of MIS-C in patients who were assessed and treated for MIS-C but did not fully meet the CDC criteria; and asymptomatic SARS-CoV-2, SARS-CoV-2+ PCR test without symptoms of COVID-19 (listed above) and admitted with alternative diagnoses. Patients with asymptomatic SARS-CoV-2 were included primarily as a comparison group.
Clinically apparent thrombosis was defined as a radiologically confirmed arterial or venous thrombus. Superficial vein thrombosis was not included. In patients with thrombosis, data on clot location, symptoms, and anticoagulation treatment were collected. Bleeding was categorized as major or clinically relevant nonmajor based on the International Society of Thrombosis and Haemostasis consensus definition.24 COVID-19–directed therapies included systemic steroids, convalescent plasma, remdesivir, tocilizumab, hydroxycholoroquine, and anakinra. MIS-C–directed therapies included systemic steroids, IV immunoglobulin, anakinra, tocilizumab, and aspirin. Type, duration, and dosing of postdischarge thromboprophylaxis were collected. Laboratory parameters were collected as maximum and/or minimum values during hospitalization and included platelet count, hemoglobin, PT, lupus anticoagulant, D-dimer, and fibrinogen.
Obesity was defined using the CDC definition for body mass index ≥95th percentile for age for children age >24 months.25 If height and weight were not available, the patient was classified as obese if documented as such in the patient’s medical record.
Statistical analysis
Standard statistical methods were used to summarize the data: frequency and percentage for categorical variables, and median and interquartile range for continuous scaled variables. The incidence of TEs was calculated, along with the 95% confidence interval (CI) for each subgroup (COVID-19, MIS-C/MIS-C like, and asymptomatic SARS-CoV-2). Comparisons of demographic and clinical characteristics among patients with and without thrombosis were performed using nonparametric statistical tests. For univariable analyses, categorical variables were analyzed using count and percentage with Fisher’s exact test, and continuous variables were analyzed using median, interquartile range, and Kruskal-Wallis rank test. Missing values were excluded from the analyses.
Multivariable analysis was performed using a binomial logit model with Wald test statistics. Given the low event rate (TEs), we only carried through a subset of the variables from the univariable analysis based on those that we believed to be independently associated. The primary analysis included patients with COVID-19 or MIS-C/MIS-C like. Because of the low number of TEs, these groups were combined, and asymptomatic SARS-CoV-2 cases were excluded. A sensitivity analysis was performed excluding MIS-C–like patients.
All calculated P values were 2 sided, and an α level of 0.05 was used for assessing significance; all analyses were conducted using SAS software (version 9.4).
Results
Between 1 March and 15 August 2020, we identified 853 hospital admissions in 814 patients meeting eligibility criteria. Of these, 426 admissions (50%) were for COVID-19, 138 (16%) for MIS-C (n = 130) or MIS-C–like illness (n = 8), and 289 (34%) for asymptomatic SARS-CoV-2 infection. Of the 8 patients who did not meet full MIS-C criteria, 1 did not have documented fever, 5 did not have SARS-CoV-2+ test or known exposure, and 2 had an alternative diagnosis. All had other features highly suggestive of MIS-C and were treated for MIS-C with steroids, IV immunoglobulin, aspirin, anakinra, and/or infliximab. Demographic and clinical characteristics of the cohort including therapies received are listed in Table 1 by clinical subgroup. For all subsequent analyses, MIS-C–like patients were combined with MIS-C patients. The MIS-C cohort had the highest proportion of patients without underlying comorbidities, with more evidence of coagulopathy (elevated D-dimer, fibrinogen, and PT and lower platelet count), and who required critical care (Table 1).
Clinical characteristics of all 3 SARS-CoV-2 cohorts
| . | Asymptomatic SARS-CoV-2 (n = 289) . | COVID-19 (n = 426) . | MIS-C/ MIS-C like (n = 138) . |
|---|---|---|---|
| Age, y | |||
| Median | 10 | 10 | 10 |
| IQR | 3-15 | 1-16 | 6-14 |
| Male sex | 144 (50) | 226 (53) | 75 (54) |
| Ethnicity | |||
| Hispanic | 125 (43) | 228 (54) | 57 (41) |
| Non-Hispanic | 139 (48) | 167 (39) | 69 (50) |
| Unknown/other | 25 (9) | 31 (7) | 12 (9) |
| Race | |||
| White | 151 (52) | 234 (55) | 77 (56) |
| African American | 74 (26) | 89 (21) | 39 (28) |
| Asian | 2 (1) | 6 (1) | 4 (3) |
| Other/unknown | 62 (21) | 97 (23) | 18 (13) |
| African American race and/or Hispanic ethnicity | 198 (69) | 315 (74) | 96 (70) |
| Length of stay, d | |||
| Median | 3 | 3 | 6 |
| IQR | 1-7 | 2-7 | 4-10 |
| Comorbidity | |||
| Respiratory | 22 (8) | 90 (21) | 16 (12) |
| Cardiac | 13 (5) | 38 (9) | 5 (4) |
| Cancer | 25 (9) | 39 (9) | 5 (4) |
| Obesity | 61 (21) | 114 (27) | 45 (33) |
| Diabetes | 17 (6) | 15 (4) | 0 |
| Immunodeficiency | 10 (3) | 18 (4) | 1 (1) |
| Sickle cell disease | 8 (3) | 26 (6) | 1 (1) |
| Neurologic | 38 (13) | 49 (12) | 4 (3) |
| Gastrointestinal | 36 (12) | 38 (9) | 1 (1) |
| None | 77 (27) | 114 (27) | 70 (51) |
| ICU admission | 46 (16) | 136 (32) | 86 (62) |
| Ventilator | 11 (4) | 46 (11) | 22 (16) |
| ECMO | 1 (0.4) | 4 (1) | 3 (2) |
| CVC | 47 (16) | 74 (17) | 53 (38) |
| COVID-19–directed treatment | 0 | 105 (25) | 39 (28) |
| MIS-C–directed treatment | 1 (0.3) | 6 (1) | 130 (94) |
| Maximum D-dimer | |||
| Not done | 214 (74) | 197 (46) | 5 (4) |
| Normal range | 14 (5) | 36 (8) | 1 (1) |
| 1-5× ULN | 34 (12) | 131 (31) | 32 (23) |
| >5× ULN | 27 (9) | 62 (15) | 100 (72) |
| Minimum platelet count, ×103/μL | |||
| Median | 259 | 208 | 148 |
| IQR | 199-320 | 140-273 | 103-204 |
| Maximum fibrinogen, mg/dL | |||
| Median | 421 | 463 | 551 |
| IQR | 282-583 | 325-583 | 462-657 |
| Maximum PT | |||
| Not done | 183 (63) | 204 (48) | 7 (5) |
| Normal range | 55 (19) | 121 (28) | 38 (27) |
| 1-1.5× ULN | 48 (17) | 90 (21) | 86 (62) |
| >1.5× ULN | 3 (1) | 11 (3) | 7 (5) |
| Thromboprophylaxis | 31 (11) | 128 (30) | 80 (58) |
| Admission with TEs | 2 (0.7) | 9 (2) | 9 (7) |
| DVT | 2 | 4 | 7 |
| PE | 0 | 3 | 0 |
| Stroke | 0 | 0 | 1 |
| Intracardiac | 0 | 2 | 1 |
| Cerebral sinovenous thrombosis | 0 | 1 | 0 |
| Major bleeding | 4 (1) | 7 (2) | 2 (1) |
| Death during hospitalization (or <30 d) | 2 (0.7) | 11 (2.6) | 2 (1.4) |
| . | Asymptomatic SARS-CoV-2 (n = 289) . | COVID-19 (n = 426) . | MIS-C/ MIS-C like (n = 138) . |
|---|---|---|---|
| Age, y | |||
| Median | 10 | 10 | 10 |
| IQR | 3-15 | 1-16 | 6-14 |
| Male sex | 144 (50) | 226 (53) | 75 (54) |
| Ethnicity | |||
| Hispanic | 125 (43) | 228 (54) | 57 (41) |
| Non-Hispanic | 139 (48) | 167 (39) | 69 (50) |
| Unknown/other | 25 (9) | 31 (7) | 12 (9) |
| Race | |||
| White | 151 (52) | 234 (55) | 77 (56) |
| African American | 74 (26) | 89 (21) | 39 (28) |
| Asian | 2 (1) | 6 (1) | 4 (3) |
| Other/unknown | 62 (21) | 97 (23) | 18 (13) |
| African American race and/or Hispanic ethnicity | 198 (69) | 315 (74) | 96 (70) |
| Length of stay, d | |||
| Median | 3 | 3 | 6 |
| IQR | 1-7 | 2-7 | 4-10 |
| Comorbidity | |||
| Respiratory | 22 (8) | 90 (21) | 16 (12) |
| Cardiac | 13 (5) | 38 (9) | 5 (4) |
| Cancer | 25 (9) | 39 (9) | 5 (4) |
| Obesity | 61 (21) | 114 (27) | 45 (33) |
| Diabetes | 17 (6) | 15 (4) | 0 |
| Immunodeficiency | 10 (3) | 18 (4) | 1 (1) |
| Sickle cell disease | 8 (3) | 26 (6) | 1 (1) |
| Neurologic | 38 (13) | 49 (12) | 4 (3) |
| Gastrointestinal | 36 (12) | 38 (9) | 1 (1) |
| None | 77 (27) | 114 (27) | 70 (51) |
| ICU admission | 46 (16) | 136 (32) | 86 (62) |
| Ventilator | 11 (4) | 46 (11) | 22 (16) |
| ECMO | 1 (0.4) | 4 (1) | 3 (2) |
| CVC | 47 (16) | 74 (17) | 53 (38) |
| COVID-19–directed treatment | 0 | 105 (25) | 39 (28) |
| MIS-C–directed treatment | 1 (0.3) | 6 (1) | 130 (94) |
| Maximum D-dimer | |||
| Not done | 214 (74) | 197 (46) | 5 (4) |
| Normal range | 14 (5) | 36 (8) | 1 (1) |
| 1-5× ULN | 34 (12) | 131 (31) | 32 (23) |
| >5× ULN | 27 (9) | 62 (15) | 100 (72) |
| Minimum platelet count, ×103/μL | |||
| Median | 259 | 208 | 148 |
| IQR | 199-320 | 140-273 | 103-204 |
| Maximum fibrinogen, mg/dL | |||
| Median | 421 | 463 | 551 |
| IQR | 282-583 | 325-583 | 462-657 |
| Maximum PT | |||
| Not done | 183 (63) | 204 (48) | 7 (5) |
| Normal range | 55 (19) | 121 (28) | 38 (27) |
| 1-1.5× ULN | 48 (17) | 90 (21) | 86 (62) |
| >1.5× ULN | 3 (1) | 11 (3) | 7 (5) |
| Thromboprophylaxis | 31 (11) | 128 (30) | 80 (58) |
| Admission with TEs | 2 (0.7) | 9 (2) | 9 (7) |
| DVT | 2 | 4 | 7 |
| PE | 0 | 3 | 0 |
| Stroke | 0 | 0 | 1 |
| Intracardiac | 0 | 2 | 1 |
| Cerebral sinovenous thrombosis | 0 | 1 | 0 |
| Major bleeding | 4 (1) | 7 (2) | 2 (1) |
| Death during hospitalization (or <30 d) | 2 (0.7) | 11 (2.6) | 2 (1.4) |
Data presented as n (%).
CVC, central venous catheter; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; PE, pulmonary embolism; ULN, upper limit of normal.
TEs
There were 20 patients with ≥1 TE; 9 patients were admitted with COVID-19, 9 with MIS-C, and 2 with asymptomatic SARS-CoV-2. One patient admitted with COVID-19 developed a second TE during the admission (total, 21 TEs). Patients with MIS-C had the highest incidence at 6.5% (95% CI, 3% to 12%), followed by those with COVID-19 at 2.1% (95% CI, 1% to 4%) and those with asymptomatic SARS-CoV-2 at 0.7% (95% CI, 0.1% to 2.4%). A majority of TEs (89%) in COVID-19 or MIS-C patients occurred in those age ≥12 years. The incidence of TEs in patients age ≥12 years with COVID-19 or MIS-C was 6.8% (16 of 237), compared with 0.6% (2 of 327) in those age <12 years. In the subgroup of patients with MIS-C who were age ≥12 years, the incidence was 19% (9 of 48). Clinical details regarding patients with thrombosis are listed in Table 2. Of the 19 TEs in patients with COVID-19 or MIS-C, there were 11 deep vein thromboses, 3 pulmonary embolisms, 3 intracardiac thromboses, 1 acute ischemic stroke, and 1 cerebral sinovenous thrombosis. Four TEs were clinically apparent on admission to the hospital (Table 2). Thirteen (68%) were symptomatic, while the rest were asymptomatic, detected on imaging performed for another indication. Nine (47%) were catheter related. Death resulting from any cause occurred in 5 (28%) of the 18 patients with COVID-19 or MIS-C and thrombosis. A vast majority of COVID-19 and MIS-C patients with thrombosis (17 of 18) identified as being of either Hispanic ethnicity or African American race. One COVID-19 patient was diagnosed with COVID toes, but this was not included in overall TEs.
Clinical characteristics of patients who developed thrombosis
| Patient . | Age, y* . | Sex . | Race/ethnicity . | Thrombosis location . | Catheter related . | Underlying medical conditions . | Thromboprophylaxis . | ICU . | Other† . |
|---|---|---|---|---|---|---|---|---|---|
| COVID-19 | |||||||||
| 1 | 16-18 | F | White/Hispanic | PE | No | Cancer (relapsed sarcoma), CVC, obesity | Enoxaparin 30 mg every 12 h | Yes | Critically ill; died as a result of cardiac arrest, COVID-19 pneumonia, hemothorax |
| 2 | 16-18 | M | African American | Upper-extremity DVT | Yes | Congenital heart disease, cerebral palsy, CVC, bacterial tracheitis | Enoxaparin every 12 h, goal anti-Xa 0.2-0.5 IU/mL | Yes | Critically ill; died as a result of cardiac arrest |
| 3 | 18-21 | M | White/Hispanic | PE | No | Cancer (AML), CVC | No; thrombosis present on admission | Yes | Critically ill; died as a result of intracranial hemorrhage, COVID-19, multisystem organ failure |
| 4 | 16-18 | M | White/Hispanic | Lower-extremity DVT | No | Obesity | No | Yes | |
| 5 | <1 | F | White/Hispanic | Intracardiac thrombosis | Yes | Cancer (AML), CVC | No | Yes | Died as a result of AML |
| 6 | 10-12 | F | African American | PE | No | Obesity | Enoxaparin 0.5 mg/kg every 12 h | Yes | Critically ill |
| 7 | 12-14 | M | White/Hispanic | Cerebral sinovenous thrombosis | No | Cancer | No | No | Major bleeding event on anticoagulation |
| 8 | 14-16 | F | African American | Upper-extremity DVT ×2 | Yes | Cancer, obesity, CVC | No; thrombosis present on admission, second thrombosis occurred while on enoxaparin 1 mg/kg every 12 h | No | |
| 9 | 14-16 | M | Other/Hispanic | Intracardiac thrombosis | Yes | Cancer, CVC | No; thrombosis present on admission | No | Readmitted after asymptomatic SARS-CoV-2 admission with symptomatic COVID-19, thrombosis on chest CT |
| MIS-C | |||||||||
| 10 | 16-18 | M | African American | Acute ischemic stroke | No | Neurologic disorder, CVC | No; stroke on admission | Yes | Critically ill; admitted with acute MCA stroke and MIS-C 1 mo after acute COVID-19 admission |
| 11 | 12-14 | F | African American | Extremity DVT | No | Respiratory, CVC | No | Yes | Critically ill; admitted in shock and with decreased cardiac function |
| 12‡ | 14-16 | F | Unknown | Lower-extremity DVT | No | None, CVC | Enoxaparin every 12 h, goal anti-Xa 0.3-0.5 IU/mL | Yes | Critically ill, on ECMO |
| 13 | 16-18 | M | African American | Lower-extremity DVT | No | None, CVC | UFH, goal anti-Xa 0.1-0.3 IU/mL | Yes | Critically ill |
| 14 | 16-18 | M | Unknown/Hispanic | Intracardiac thrombosis | Yes | Obesity, CVC | UFH, goal anti-Xa 0.3-0.7 IU/mL | Yes | Critically ill; thrombosis found post-ECMO |
| 15 | 16-18 | F | African American | Upper-extremity DVT | Yes | Obesity, CVC | Heparin 5000 units every 12 h then enoxaparin, goal anti-Xa 0.5-1.0 IU/mL | Yes | Critically ill |
| 16‡ | 14-16 | F | White/Hispanic | Upper-extremity DVT | Yes | Cancer, obesity, respiratory, CVC | Enoxaparin daily | No | Died as a result of multiorgan failure |
| 17‡ | 18-21 | M | African American | Upper-extremity DVT | No | Cancer, obesity, CVC | Enoxaparin, goal anti-Xa 0.3-0.5 IU/mL | No | Symptomatic COVID-19 then readmitted with MIS-C, relapsed ALL, bacteremia |
| 18 | 16-18 | F | African American | Upper-extremity DVT | Yes | Obesity, CVC | Enoxaparin, goal anti-Xa 0.2-0.5 IU/mL | No | Urinary tract infection |
| Asymptomatic SARS-CoV-2 | |||||||||
| 19 | 16-18 | F | White/Hispanic | Lower-extremity DVT | No | Cancer (ALL in induction) | Enoxaparin 40 mg daily | Yes | Died as a result of complications of ALL; bacteremia |
| 20 | <1 | F | African American | Lower-extremity DVT | Yes | Acute liver failure, CVC | No | Yes |
| Patient . | Age, y* . | Sex . | Race/ethnicity . | Thrombosis location . | Catheter related . | Underlying medical conditions . | Thromboprophylaxis . | ICU . | Other† . |
|---|---|---|---|---|---|---|---|---|---|
| COVID-19 | |||||||||
| 1 | 16-18 | F | White/Hispanic | PE | No | Cancer (relapsed sarcoma), CVC, obesity | Enoxaparin 30 mg every 12 h | Yes | Critically ill; died as a result of cardiac arrest, COVID-19 pneumonia, hemothorax |
| 2 | 16-18 | M | African American | Upper-extremity DVT | Yes | Congenital heart disease, cerebral palsy, CVC, bacterial tracheitis | Enoxaparin every 12 h, goal anti-Xa 0.2-0.5 IU/mL | Yes | Critically ill; died as a result of cardiac arrest |
| 3 | 18-21 | M | White/Hispanic | PE | No | Cancer (AML), CVC | No; thrombosis present on admission | Yes | Critically ill; died as a result of intracranial hemorrhage, COVID-19, multisystem organ failure |
| 4 | 16-18 | M | White/Hispanic | Lower-extremity DVT | No | Obesity | No | Yes | |
| 5 | <1 | F | White/Hispanic | Intracardiac thrombosis | Yes | Cancer (AML), CVC | No | Yes | Died as a result of AML |
| 6 | 10-12 | F | African American | PE | No | Obesity | Enoxaparin 0.5 mg/kg every 12 h | Yes | Critically ill |
| 7 | 12-14 | M | White/Hispanic | Cerebral sinovenous thrombosis | No | Cancer | No | No | Major bleeding event on anticoagulation |
| 8 | 14-16 | F | African American | Upper-extremity DVT ×2 | Yes | Cancer, obesity, CVC | No; thrombosis present on admission, second thrombosis occurred while on enoxaparin 1 mg/kg every 12 h | No | |
| 9 | 14-16 | M | Other/Hispanic | Intracardiac thrombosis | Yes | Cancer, CVC | No; thrombosis present on admission | No | Readmitted after asymptomatic SARS-CoV-2 admission with symptomatic COVID-19, thrombosis on chest CT |
| MIS-C | |||||||||
| 10 | 16-18 | M | African American | Acute ischemic stroke | No | Neurologic disorder, CVC | No; stroke on admission | Yes | Critically ill; admitted with acute MCA stroke and MIS-C 1 mo after acute COVID-19 admission |
| 11 | 12-14 | F | African American | Extremity DVT | No | Respiratory, CVC | No | Yes | Critically ill; admitted in shock and with decreased cardiac function |
| 12‡ | 14-16 | F | Unknown | Lower-extremity DVT | No | None, CVC | Enoxaparin every 12 h, goal anti-Xa 0.3-0.5 IU/mL | Yes | Critically ill, on ECMO |
| 13 | 16-18 | M | African American | Lower-extremity DVT | No | None, CVC | UFH, goal anti-Xa 0.1-0.3 IU/mL | Yes | Critically ill |
| 14 | 16-18 | M | Unknown/Hispanic | Intracardiac thrombosis | Yes | Obesity, CVC | UFH, goal anti-Xa 0.3-0.7 IU/mL | Yes | Critically ill; thrombosis found post-ECMO |
| 15 | 16-18 | F | African American | Upper-extremity DVT | Yes | Obesity, CVC | Heparin 5000 units every 12 h then enoxaparin, goal anti-Xa 0.5-1.0 IU/mL | Yes | Critically ill |
| 16‡ | 14-16 | F | White/Hispanic | Upper-extremity DVT | Yes | Cancer, obesity, respiratory, CVC | Enoxaparin daily | No | Died as a result of multiorgan failure |
| 17‡ | 18-21 | M | African American | Upper-extremity DVT | No | Cancer, obesity, CVC | Enoxaparin, goal anti-Xa 0.3-0.5 IU/mL | No | Symptomatic COVID-19 then readmitted with MIS-C, relapsed ALL, bacteremia |
| 18 | 16-18 | F | African American | Upper-extremity DVT | Yes | Obesity, CVC | Enoxaparin, goal anti-Xa 0.2-0.5 IU/mL | No | Urinary tract infection |
| Asymptomatic SARS-CoV-2 | |||||||||
| 19 | 16-18 | F | White/Hispanic | Lower-extremity DVT | No | Cancer (ALL in induction) | Enoxaparin 40 mg daily | Yes | Died as a result of complications of ALL; bacteremia |
| 20 | <1 | F | African American | Lower-extremity DVT | Yes | Acute liver failure, CVC | No | Yes |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CT, computed tomography; CVC, central venous catheter; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; MCA, middle cerebral artery; PE, pulmonary embolism; UFH, unfractionated heparin.
*Age range presented so patient identification less likely.
†Critically ill indicates patient required mechanical ventilation, vasopressors, and/or ECMO.
‡MIS-C like.
We evaluated several clinical, demographic, and laboratory risk factors for thrombosis in the combined cohort of COVID-19 and MIS-C, listed in Table 3. Older age, African American race and/or Hispanic ethnicity, MIS-C, admission to the ICU, need for a ventilator, length of stay, presence of a CVC, cancer, D-dimer >5× ULN, PT >1.5× ULN, elevated fibrinogen, reduced platelet count, and death during admission were all statistically significant variables on univariable analysis. In a univariable analysis specifically comparing the rate of thrombosis in COVID-19 patients vs asymptomatic SARS-CoV-2 patients, COVID-19 was not significantly associated with TEs (P = .2).
Comparison of COVID-19 or MIS-C/MIS-C–like patients with thrombosis with those without
| . | Thrombosis (n = 18) . | No thrombosis (n = 546) . | P . |
|---|---|---|---|
| Age, y | .0001 | ||
| Median | 16 | 10 | |
| IQR | 14-17 | 2-15 | |
| Age group, y | .0002 | ||
| 0-6 | 1 (6) | 209 (38) | |
| 6-12 | 1 (6) | 116 (21) | |
| ≥12 | 16 (89) | 221 (40) | |
| Male sex | 9 (50) | 292 (53) | .81 |
| Hispanic ethnicity | 8 (44) | 277 (51) | .64 |
| Race | |||
| White | 6 (33) | 305 (56) | .09 |
| African American | 9 (50) | 119 (22) | .009 |
| Asian | 0 | 10 (2) | 1 |
| Other/unknown | 3 (17) | 112 (21) | 1 |
| African American race and/or Hispanic ethnicity | 17 (94) | 394 (72) | .03 |
| SARS-CoV-2 disease category | |||
| COVID-19 | 9 (50) | 417 (76) | .02 |
| MIS-C | 9 (50) | 129 (24) | |
| Length of stay, d | <.0001 | ||
| Median | 18 | 4 | |
| IQR | 10-41 | 2-8 | |
| Comorbidity | |||
| Respiratory | 3 (17) | 103 (19) | 1 |
| Cardiac | 1 (6) | 42 (8) | 1 |
| Cancer | 8 (44) | 36 (7) | <.0001 |
| Obesity | 9 (50) | 150 (27) | .06 |
| Neurologic | 2 (11) | 51 (9) | .68 |
| Sickle cell disease | 0 | 27 | 1 |
| None | 2 (11) | 182 (33) | .07 |
| ICU admission | 13 (72) | 209 (38) | .006 |
| Ventilator | 9 (50) | 59 (11) | .004 |
| ECMO | 2 (11) | 5 (1) | .06 |
| CVC | 15 (83) | 112 (21) | <.0001 |
| Maximum D-dimer | .002 | ||
| Not done | 2 (11) | 200 (37) | |
| Normal range | 0 | 37 (7) | |
| 1-5× ULN | 2 (11) | 161 (29) | |
| >5× ULN | 14 (78) | 148 (27) | |
| Maximum fibrinogen, mg/dL | .034 | ||
| Median | 632 | 499 | |
| IQR | 449-788 | 386-602 | |
| Minimum platelet count, ×103/μL | .0001 | ||
| Median | 71 | 189 | |
| IQR | 54-152 | 131-266 | |
| PT | .03 | ||
| Not done | 1 (6) | 210 (38) | |
| Normal range | 3 (17) | 156 (29) | |
| 1-1.5× ULN | 12 (67) | 164 (30) | |
| >1.5× ULN | 2 (11) | 16 (3) | |
| Thromboprophylaxis | 10 (56) | 198 (36) | .134 |
| Bacterial coinfection | 5 (28) | 67 (12) | .07 |
| Death during hospitalization (or <30 d) | 5 (28) | 8 (1) | <.0001 |
| . | Thrombosis (n = 18) . | No thrombosis (n = 546) . | P . |
|---|---|---|---|
| Age, y | .0001 | ||
| Median | 16 | 10 | |
| IQR | 14-17 | 2-15 | |
| Age group, y | .0002 | ||
| 0-6 | 1 (6) | 209 (38) | |
| 6-12 | 1 (6) | 116 (21) | |
| ≥12 | 16 (89) | 221 (40) | |
| Male sex | 9 (50) | 292 (53) | .81 |
| Hispanic ethnicity | 8 (44) | 277 (51) | .64 |
| Race | |||
| White | 6 (33) | 305 (56) | .09 |
| African American | 9 (50) | 119 (22) | .009 |
| Asian | 0 | 10 (2) | 1 |
| Other/unknown | 3 (17) | 112 (21) | 1 |
| African American race and/or Hispanic ethnicity | 17 (94) | 394 (72) | .03 |
| SARS-CoV-2 disease category | |||
| COVID-19 | 9 (50) | 417 (76) | .02 |
| MIS-C | 9 (50) | 129 (24) | |
| Length of stay, d | <.0001 | ||
| Median | 18 | 4 | |
| IQR | 10-41 | 2-8 | |
| Comorbidity | |||
| Respiratory | 3 (17) | 103 (19) | 1 |
| Cardiac | 1 (6) | 42 (8) | 1 |
| Cancer | 8 (44) | 36 (7) | <.0001 |
| Obesity | 9 (50) | 150 (27) | .06 |
| Neurologic | 2 (11) | 51 (9) | .68 |
| Sickle cell disease | 0 | 27 | 1 |
| None | 2 (11) | 182 (33) | .07 |
| ICU admission | 13 (72) | 209 (38) | .006 |
| Ventilator | 9 (50) | 59 (11) | .004 |
| ECMO | 2 (11) | 5 (1) | .06 |
| CVC | 15 (83) | 112 (21) | <.0001 |
| Maximum D-dimer | .002 | ||
| Not done | 2 (11) | 200 (37) | |
| Normal range | 0 | 37 (7) | |
| 1-5× ULN | 2 (11) | 161 (29) | |
| >5× ULN | 14 (78) | 148 (27) | |
| Maximum fibrinogen, mg/dL | .034 | ||
| Median | 632 | 499 | |
| IQR | 449-788 | 386-602 | |
| Minimum platelet count, ×103/μL | .0001 | ||
| Median | 71 | 189 | |
| IQR | 54-152 | 131-266 | |
| PT | .03 | ||
| Not done | 1 (6) | 210 (38) | |
| Normal range | 3 (17) | 156 (29) | |
| 1-1.5× ULN | 12 (67) | 164 (30) | |
| >1.5× ULN | 2 (11) | 16 (3) | |
| Thromboprophylaxis | 10 (56) | 198 (36) | .134 |
| Bacterial coinfection | 5 (28) | 67 (12) | .07 |
| Death during hospitalization (or <30 d) | 5 (28) | 8 (1) | <.0001 |
Data presented as n (%).
CVC, central venous catheter; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; PT, prothrombin time.
Data presented as n (%).
D-dimer >5× ULN was significantly associated with TEs in the univariable analysis. However, there was a high proportion (36%) of missing D-dimer values, which was a problem for all the laboratory variables. Rather than imputing values, we ran the multivariable modeling in 2 ways: without laboratory values and including only admissions that had a D-dimer performed. In the model without laboratory values, the following variables were significantly associated with thrombosis: age ≥12 years, cancer, CVC, and MIS-C (Table 4). When D-dimer was included in the model, it was significant, but MIS-C was no longer statistically significant (Table 4).
Multivariable model for factors associated with thrombosis in COVID-19 and MIS-C/MIS-C–like patients
| . | Without D-dimer . | With D-dimer* . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P . | OR (95% CI) . | P . |
| Cancer | 6.34 (1.56-25.73) | .01 | 13.74 (2.36-79.95) | .004 |
| Obesity | 0.89 (0.24-3.25) | .856 | 1.16 (0.21-6.35) | .863 |
| No comorbidity | 0.31 (0.04-2.17) | .236 | 0.43 (0.05-3.79) | .447 |
| Bacterial coinfection | 1.63 (0.41-6.48) | .484 | 1.31 (0.27-6.43) | .741 |
| CVC | 7.22 (1.71-30.45) | .007 | 25.71 (2.65-249.01) | .005 |
| ICU admission | 2.17 (0.56-8.40) | .263 | 0.35 (0.04-3.04) | .339 |
| MIS-C | 6.44 (1.65-25.24) | .008 | 3.18 (0.57-17.55) | .185 |
| COVID-19 | Reference | Reference | ||
| Age <6 y | 1.30 (0.06-27.71) | .871 | 1.19 (0.05-30.28) | .916 |
| Age 6-12 y | Reference | Reference | ||
| Age ≥12 y | 16.84 (1.93-147.1) | .011 | 20.05 (2.18-184.29) | .008 |
| African American race and/or Hispanic ethnicity | 7.14 (0.83-61.36) | .073 | 9.74 (0.91-104.45) | .06 |
| D-dimer >5× ULN | Not included | 21.16 (2-223.94) | .011 | |
| . | Without D-dimer . | With D-dimer* . | ||
|---|---|---|---|---|
| Variable . | OR (95% CI) . | P . | OR (95% CI) . | P . |
| Cancer | 6.34 (1.56-25.73) | .01 | 13.74 (2.36-79.95) | .004 |
| Obesity | 0.89 (0.24-3.25) | .856 | 1.16 (0.21-6.35) | .863 |
| No comorbidity | 0.31 (0.04-2.17) | .236 | 0.43 (0.05-3.79) | .447 |
| Bacterial coinfection | 1.63 (0.41-6.48) | .484 | 1.31 (0.27-6.43) | .741 |
| CVC | 7.22 (1.71-30.45) | .007 | 25.71 (2.65-249.01) | .005 |
| ICU admission | 2.17 (0.56-8.40) | .263 | 0.35 (0.04-3.04) | .339 |
| MIS-C | 6.44 (1.65-25.24) | .008 | 3.18 (0.57-17.55) | .185 |
| COVID-19 | Reference | Reference | ||
| Age <6 y | 1.30 (0.06-27.71) | .871 | 1.19 (0.05-30.28) | .916 |
| Age 6-12 y | Reference | Reference | ||
| Age ≥12 y | 16.84 (1.93-147.1) | .011 | 20.05 (2.18-184.29) | .008 |
| African American race and/or Hispanic ethnicity | 7.14 (0.83-61.36) | .073 | 9.74 (0.91-104.45) | .06 |
| D-dimer >5× ULN | Not included | 21.16 (2-223.94) | .011 | |
CVC, central venous catheter; OR, odds ratio; ULN, upper limit of normal
Missing values excluded.
In a sensitivity analysis that excluded the 8 MIS-C–like patients, the results of the multivariable analyses were similar to those of the primary analysis (data not shown).
Thromboprophylaxis
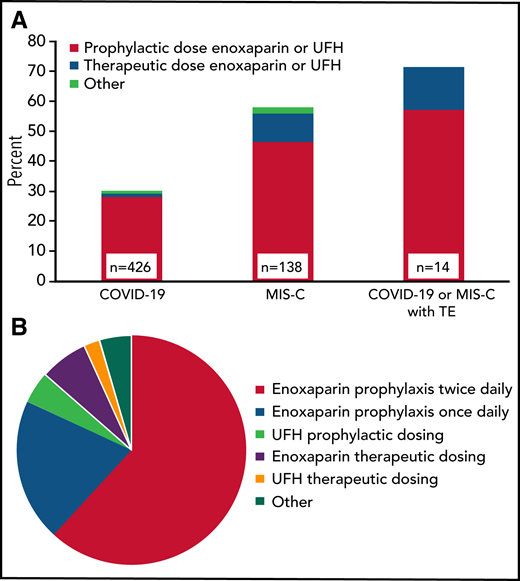
Anticoagulant thromboprophylaxis was used during 30% (128 of 426) of COVID-19 and 58% (80 of 138) of MIS-C admissions, as well as during 61% of ICU admissions in those patients (Figure 1A). Nonetheless, 10 (71%) of the 14 TEs that were not identified on admission occurred in patients receiving thromboprophylaxis (regimens listed in Table 2). There were 220 regimens used in the 208 COVID-19 or MIS-C admissions where prophylactic anticoagulation was administered. The most common anticoagulant was enoxaparin (89%), followed by unfractionated heparin in 6.8% and other anticoagulants (4.5%). The variation with respect to drug and intensity is shown in Figure 1B. Once daily enoxaparin accounted for 20%, whereas a majority (62%) received twice daily prophylactic enoxaparin (∼0.5 mg/kg every 12 hours). Full therapeutic intensity prophylaxis with enoxaparin (∼1 mg/kg every 12 hours) or unfractionated heparin was used in 9.1%.
Prophylactic anticoagulation regimens in COVID-19 and MIS-C patients. (A) Proportion of admissions receiving prophylactic anticoagulation in clinical subgroups by dose intensity (n = 208 admissions). When >1 regimen was used per admission, the regimen with either the longest duration or highest dose intensity was included. For patients with TEs, only those without TEs on admission were included (n = 14). (B) Prophylactic anticoagulation regimens in patients with COVID-19 or MIS-C (n = 220 regimens; other: rivaroxaban, apixaban, bivalirudin, warfarin, or aspirin). TE, thrombotic event; UFH, unfractionated heparin.
Prophylactic anticoagulation regimens in COVID-19 and MIS-C patients. (A) Proportion of admissions receiving prophylactic anticoagulation in clinical subgroups by dose intensity (n = 208 admissions). When >1 regimen was used per admission, the regimen with either the longest duration or highest dose intensity was included. For patients with TEs, only those without TEs on admission were included (n = 14). (B) Prophylactic anticoagulation regimens in patients with COVID-19 or MIS-C (n = 220 regimens; other: rivaroxaban, apixaban, bivalirudin, warfarin, or aspirin). TE, thrombotic event; UFH, unfractionated heparin.
Notably, 45 (11%) of 417 patients with COVID-19 and 32 (25%) of 129 with MIS-C who did not develop thrombosis were discharged on prophylactic anticoagulation. A majority were discharged on enoxaparin (n = 56), followed by direct oral anticoagulants (n = 16), aspirin (n = 4), and warfarin (n = 1). The only patient known to develop thrombosis after discharge was a patient with cancer, found to have a catheter-associated intracardiac thrombosis within 30 days after an admission with asymptomatic SARS-CoV-2 when he was readmitted for COVID-19; this patient had not been discharged on anticoagulation.
Bleeding
There were 9 admissions with major bleeding and 8 with clinically relevant nonmajor bleeding among the COVID-19 and MIS-C subgroups (Table 1). The COVID-19 cohort had 7 admissions (1.6%) with major bleeding events, 2 of which occurred while on anticoagulation, and there were 2 (1.4%) in the MIS-C cohort with major bleeding (both events occurred on anticoagulation). Of the 4 COVID-19 or MIS-C patients with major bleeding on anticoagulation, 1 was receiving prophylactic daily enoxaparin, 1 was receiving therapeutic-dose unfractionated heparin for extracorporeal membrane oxygenation and treatment of TEs, and 2 were receiving therapeutic-dose enoxaparin for treatment of TEs.
Mortality
The all-cause mortality rate in our cohort of COVID-19 and MIS-C patients was 2.3% (13 of 564). A majority of deaths (n = 11) occurred in patients hospitalized with COVID-19. All patients who died had an underlying comorbidity; the most common was cancer (n = 7). Respiratory failure was the most common reason for death (n = 7). The cause of death was attributed, at least in part, to malignancy in 3 patients. One patient had a hemothorax while on therapeutic anticoagulation that was thought to contribute to death. Other reported contributors to death included renal failure (n = 1), thrombotic microangiopathy (n = 1), complex congenital heart disease (n = 1), bowel obstruction with abdominal compartment syndrome (n = 1), and septic shock (n = 1). Five of the 18 patients (28%) with COVID-19 or MIS-C and TEs died.
Discussion
This is the first study to evaluate the incidence of thrombosis in a large, multicenter cohort of children hospitalized with COVID-19–related complications.16,26-28 The incidence of TEs in hospitalized children was 2.1% in those with COVID-19 and 6.5% in those with MIS-C, compared with 0.7% in those with asymptomatic SARS-CoV-2 infection. We identified several important laboratory (elevated D-dimer) and clinical variables (age ≥12 years, cancer, MIS-C, and CVC) that were associated with a much higher risk of TEs. Importantly, more than two-thirds of TEs that occurred in hospitalized children with COVID-19 or MIS-C occurred in patients receiving thromboprophylaxis. These findings have important implications for clinicians and provide new evidence regarding the risk of TEs in children hospitalized with COVID-19 or MIS-C. Mortality was 28% in pediatric patients with TEs and COVID-19 or MIS-C, although most patients who died had comorbid conditions that were risk factors for TEs and contributed to mortality.10
To put our findings into perspective, the rate of VTE in children admitted to US tertiary care hospitals in 2007 was estimated at 58 (0.58%) per 10 000 admissions using the Pediatric Health Information System database, with those age 12 to 18 years having the highest rate (94 [0.94%] per 10 000).29 The rate observed in our study was much higher, particularly in patients age ≥12 years (6.8%) with COVID-19 or MIS-C. However, this comparison is limited for several reasons. Patients hospitalized with COVID-19 are known to have a high prevalence of underlying medical conditions (eg, cancer, diabetes, and obesity), which may have increased the rate. In addition, the age distribution of patients in this study was skewed toward older patients compared with the Pediatric Health Information System study, which would also result in a higher rate. Nonetheless, the rates of TEs that we observed, particularly in the MIS-C population and those age ≥12 years, suggest that COVID-19 and MIS-C are unique risk factors for thrombosis in hospitalized children.
Consistent with this conclusion is the finding that a D-dimer >5× ULN was significantly associated with TEs in our study. This is the first pediatric COVID-19 study to demonstrate the association between elevated D-dimer and TEs, and this finding is consistent with multiple studies of adults with COVID-19.8,30,31 In addition, elevated fibrinogen, prolonged PT, and reduced platelet count were also associated with TEs in our study on univariable analysis, similar to reports of adults with COVID-19.1-3 A limitation of our analysis is that there was a high proportion of missing laboratory values, and we limited the collection of laboratory parameters to the maximum or minimum values during hospitalization, regardless of timing (ie, early in hospitalization and/or before TEs). Therefore, it is possible that elevations in D-dimer levels may have occurred after a TE in some patients, reducing the predictive value of this variable. Future studies will be necessary to better evaluate the predictive value of elevated D-dimer in this setting.
In a multivariable regression model that excluded laboratory values, MIS-C, age ≥12 years, presence of a CVC, and cancer were significantly associated with TEs. With the exception of MIS-C, these mirror previously established risk factors for TEs in hospitalized children.32,33 When D-dimer was included in the multivariable model, it was significant; however, MIS-C was no longer significant. We hypothesize that this is due to the collinearity of these variables, as well as to the proportion of missing laboratory values. Interestingly, cancer was identified as an independent risk factor for TEs in multivariable models with and without D-dimer. Although COVID-19 was not found to be a risk factor for TEs, 30% of patients with COVID-19 received thromboprophylaxis, which may have reduced the rate of thrombosis in this group. In addition, our ability to evaluate COVID-19 as a risk factor may have been limited by the small sample size. Because of the small sample size, missing laboratory values, and lack of a validation cohort, we did not develop a clinical prediction model. Future studies, focused on TE risk prediction in children with SARS-CoV-2, should address these points and also include other potentially important variables, such as blood type and pubertal status (rather than age).
We decided to include asymptomatic patients with SARS-CoV-2 for several reasons. First, we wanted to evaluate the risk of thrombosis in these children, because hematologists are frequently consulted to address the role of thromboprophylaxis in this setting. Second, if patients were truly asymptomatic and had no effects from viremia, this group could potentially serve as a control group. The overall incidence of TEs in the asymptomatic SARS-CoV-2 group was low (0.7%), and the 2 patients with TEs in this subgroup had several prothrombotic risk factors (Table 2). Therefore, we believe that asymptomatic SARS-CoV-2 infection may not significantly increase the risk of thrombosis.
In our study, all 9 of the TEs in the MIS-C cohort occurred in patients age ≥12 years, for a rate of 19% (9 of 48) in this subgroup. This is higher than that in an earlier report from a multicenter registry of 186 MIS-C patients, where 3 (6.7%) of 45 patients age >12 years had TEs.16 This difference may be due to variation in thromboprophylaxis, severity of disease, inclusion of different centers, or because our study was designed to focus on TEs. We did not identify any TEs in the 90 MIS-C admissions that occurred in children age <12 years, confirming the low incidence of TEs in younger children with MIS-C observed in the prior study.
Similar to other studies, we observed a high prevalence of Hispanic (51%) and African American children (23%) hospitalized with COVID-19 or MIS-C.34 Of the 18 patients with TEs and COVID-19 or MIS-C, 17 identified as being of either Hispanic ethnicity or African American race. These racial disparities associated with worse outcomes in both children and adults are likely linked to similar socioeconomic disadvantages that have been suggested by others.12,34,35
A majority of TEs in our study developed while patients were receiving thromboprophylaxis, which is similar to adult reports.7,8 This has raised questions regarding the optimal intensity of prophylactic anticoagulation for patients with SARS-CoV-2. We are unable to answer this question because thromboprophylactic practices varied across institutions and changed during the study period. However, it is notable that 4 of 9 of the major bleeding events in patients with COVID-19 and MIS-C occurred on anticoagulation. This is limited by the fact that we could not assess all clinical risk factors for bleeding in these patients; however, anticoagulation likely contributed to these bleeding events. Optimal thromboprophylactic dose intensity may be more carefully addressed by an ongoing clinical trial to evaluate the safety of enoxaparin in children with SARS-CoV-2–associated illness, administered twice daily to achieve a 4-hour postdose anti–Xa level of 0.20 to 0.49 IU/mL.36
There are several limitations to this retrospective study. First, our findings must be contextualized, recognizing that a significant proportion of patients received inpatient and postdischarge thromboprophylaxis. Given the lack of a comparator group, it is plausible that the rate of thrombosis in this cohort would have been higher in the absence of thromboprophylaxis. Second, participating hospitals were large pediatric referral centers, and patients may have had more medically complex cases or been transferred from outside hospitals for a higher level of care. Thus, the risk of thrombosis may actually be an overestimate, and results from our study may not be generalizable to smaller pediatric centers or community hospitals. In contrast, some patients may have died as a result of unrecognized TEs or PE; this number is likely low, but would have resulted in an underestimate of the risk. Third, there was some variation over time regarding the practice of testing all admitted patients for SARS-CoV-2 (particularly earlier in the pandemic), so patients with mild disease may have been missed. Last, is important to note that a proportion of patients in this study (estimated at <10%) have been reported in prior publications or included in other registries.16,37-39 To our knowledge, none of the TEs have been previously reported.
For the first time, we provide data regarding the rate of thrombotic complications in patients age <21 years hospitalized with SARS-CoV-2–associated illness. Our findings, including the low rate of TEs in children age <12 years with COVID-19 or MIS-C and the low rate of postdischarge TEs, along with factors associated with an increased risk (age ≥12 years, MIS-C, CVC, and cancer), may help inform thromboprophylactic strategies at pediatric centers. Future studies focused on the intensity of anticoagulation as well as on novel targets are well under way in adults with COVID-19 and will likely be relevant to high-risk adolescent patients with COVID-19 or MIS-C, in whom the rates of thrombosis and mortality were highest.
For original data, please contact raffini@chop.edu.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant T32 HL007971 (H.W.) and a scholarship from the Institute for Translational Medicine and Therapeutics at the University of Pennsylvania (C.D.).
Authorship
Contribution: H.W., S.E.S., R.K., K.A., L.B., M.B., C.T.C., R.D., C.D., N.A.G., J.J., A.G.R., S.R.-Z., A.S., L.S., A.Z., and L.R. contributed to study design; H.W., S.E.S., R.K., K.A., M.B., C.T.C., R.D., C.D., J.J., J.K., K.M., S.R.-Z., W.S.L., A.S., L.S., A.Z., and L.R. contributed to data collection; H.W., L.B., and L.R. analyzed data; and H.W., S.E.S., R.K., K.A., L.B., M.B., C.T.C., R.D., C.D., N.A.G., J.J., A.G.R., S.R.-Z., A.S., L.S., A.Z., and L.R. wrote and edited the paper.
Conflict-of-interest disclosure: S.E.S. serves on the advisory board for Alexion Pharmaceuticals. R.K. has attended advisory board meetings for Bayer, Genentech, and Kedrion. The remaining authors declare no competing financial interests.
Correspondence: Leslie Raffini, 11022 Colkett Translational Research Building, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: raffini@chop.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal