In this issue of Blood, Alameda et al provide a transcriptomic atlas of successive stages of normal plasma cell development. They show that plasma cells from patients with light-chain amyloidosis (AL) most closely resemble secondary lymphoid organ plasma cells (SLO-PCs), whereas multiple myeloma (MM) cells are closer to peripheral blood plasma cells (PB-PCs) and newborn bone marrow plasma cells (BM-PCs).1
AL and MM share a common causal root, namely, the presence of clonal malignant plasma cells in the bone marrow, but the behavior of these cells differ, leading to profoundly different clinical consequences. In the first case, the clonal plasma cells do not accumulate but induce the deposition of light chains in various organs as amyloid fibrils. The deposition of amyloid fibrils is independent of the tumor burden, which is true for all monoclonal gammopathies of clinical significance.2 In the case of MM, clonal plasma cells accumulate in the bone marrow, leading to anemia and bone destruction, among other harmful effects. So far, attempts to explain why these differences occur have not been very successful. t(11;14) is more common in AL compared with MM (∼60% vs 20%). However, it is also seen in plasma cell leukemia, making 3 strikingly different disorders with the same translocation. This important finding prompted the evaluation of venetoclax for the treatment of AL.3 The same Navarra team and others have recently shown that the genomic landscape of AL is characterized by the same heterogeneity as that of MM. None of the few differences observed in terms of mutations and copy number abnormalities were sufficient to explain the clinical presentation of amyloidosis, suggesting that the explanation may not lie in the biology of the tumor plasma cell.4,5 A return to physiology of plasma cell development was clearly warranted .
In this issue of Blood, Alameda et al performed RNA sequencing of SLO-PCs, PB-PCs, and BM-PCs from healthy adults and observed 13 transcriptional programs (TPs) during the migration and transition of plasma cells through SLO, PB, and BM. This atlas is a wealth of information for researchers exploring the physiology of long-lived immunoglobulin-producing plasma cells, including unprecedented data on the ectoenzyme CD39 as a discriminatory biomarker of newborn BM-PCs.
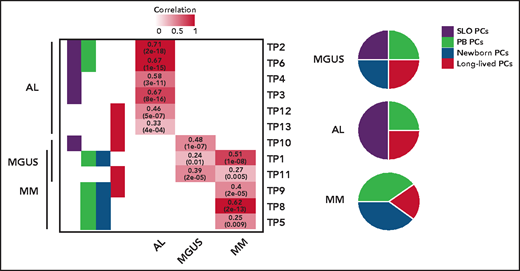
The origin of monoclonal gammopathies is an old and never resolved debate: is it circulating postgerminal center B cells or long-lived BM-PCs? By comparing these physiological TPs, the authors show that tumor PCs of 46 MM, 37 AL, and 6 monoclonal gammopathies of undetermined significance express signatures from SLO-PCs, PB-PCs, newborn BM-PCs, and long-lived BM-PCs. However, a predominant TP from SLO-PCs was expressed in AL, and from both PB-PCs and newborn BM-PCs in MM (see figure). What about at the cellular level? With Wang's recent study, we are here faced with the first available pieces of data of single-cell RNAseq data in AL.6 As in MM, the authors observed a predominant TP expressed by many tumors PCs, but with significant single-cell diversity because most described TPs were detectable in small numbers of tumor PCs. This demonstrates once again the extraordinary organization of this tumor, a kind of army made up of different infantry regiments specialized in all useful areas (invasion, survival, etc). The regiment that predominates at the time of analysis (snapshot of a dynamic process) has been selected for the needs of a given battle with the enemy. Here, however, there is no question of treatment as yet, because we are only dealing with patients at diagnosis; the only enemy to possibly fight is the antitumoral microenvironment. However, it is easy to imagine that chemotherapy could favor the selection of a resistance TP, as described in melanoma.7 The next step could be to identify underlying (epigenetic?) mechanisms of this transcriptional diversity. Once again, what single-cell sequencing technology is showing us makes us dizzy by suggesting that when the transcriptome seems to change in the bulk of the tumor, the reality may be the selection of a minority clone that was already expressing the needed transcriptome. So, like a Swiss Army knife, plasma cell disorders come equipped with all the tools needed to fend off the chemotherapy onslaught used to try to defeat it.
TPs related to normal PCs differentiation assigned to tumor PCs from patients with AL, MM, and monoclonal gammopathy of undetermined significance (MGUS), based on significant correlation levels. The numerical distribution of the TPs is represented in disease-specific pie charts. See the complete supplemental Figure 1B-C in the article by Alameda et al that begins on page 1583.
TPs related to normal PCs differentiation assigned to tumor PCs from patients with AL, MM, and monoclonal gammopathy of undetermined significance (MGUS), based on significant correlation levels. The numerical distribution of the TPs is represented in disease-specific pie charts. See the complete supplemental Figure 1B-C in the article by Alameda et al that begins on page 1583.
However, why is amyloidosis not MM? This study paves the way to answering this question, notably by highlighting the role of overexpression of genes linked to protein N-linked glycosylation (physiologically turned off when SLO-PCs egress to PB) and downregulation of genes related to ribosomes biogenesis (physiologically turned on when SLO-PCs egress to PB). Finally, Alameda et al wandered if predominant TP of tumor cells can translate into the clinic. Data presented suggest that patients with AL and MM whose tumors cells are enriched with immature profile display inferior outcome, independently of high-risk cytogenetic. Even if this work will not definitively close the old debate about origin of gammopathies, it undoubtedly brings some new insight into how cell of origin influences clinical behavior of tumor, as in the well-known case of chronic lymphocytic leukemia.8,9
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal