In this issue of Blood, Dhakal and colleagues report on the antibody response in patients receiving COVID-19 vaccination after cellular therapy.1
Emmanuel Kant once said that intelligence can be measured by the amount of uncertainty that one can bear. The COVID-19 pandemic is a marathon on quicksand, where health care professionals have to move before they sink and do not have the luxury of waiting for much solid validation, before taking action.
Because of the poor prognosis of patients with SARS-CoV-2 infection who had hematological cancers, vaccination soon appeared as the only option to protect them from the risk of serious consequences from contracting the disease. Yet, seminal vaccination studies did not provide conclusive data regarding patients with malignancies and/or receiving immunosuppression. Academic societies therefore issued expert opinion recommendations, based on previous experience in other diseases, advocating vaccination in patients with cancer and cellular therapy, despite the lack of firm evidence.
Six months after the licensing of the first vaccine, our knowledge has increased exponentially, albeit advanced by studies with very limited numbers of patients. The safety of the COVID-19 vaccines in patients who have cancer has been supported, but especially those still receiving chemotherapy have shown a lower immune response compared with that of healthy individuals,.2 Patients with hematological malignancies have been shown to mount significantly lower antibody responses than those with solid tumors, with serological conversion rates ∼85% after the second vaccination and with lower responses in patient who have received anti-CD20 therapy or have undergone hematopoietic cell transplantation (HCT) or chimeric antigen receptor (CAR) T-cell therapy.3
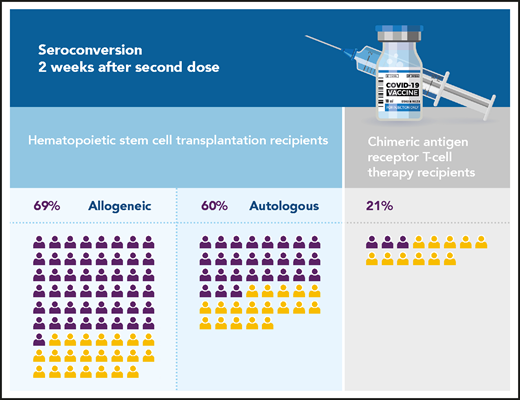
Seroconversion rates of cellular therapy recipients after the second dose of COVID-19 vaccine (the number of icons reflects the total number of patients evaluated in Dhakal et al). Purple, positive seroconversion; yellow, no seroconversion.
Seroconversion rates of cellular therapy recipients after the second dose of COVID-19 vaccine (the number of icons reflects the total number of patients evaluated in Dhakal et al). Purple, positive seroconversion; yellow, no seroconversion.
In their article, Dhakal and colleagues concentrate specifically on cellular therapy recipients. They report the antibody responses to COVID-19 vaccination of 130 HCT or CAR T-cell therapy recipients, the largest cohort of such patients to date. They show seroconversion rates of 69%, 60%, and 21% for allogeneic HCT, autologous HCT, and CAR-T recipients, respectively (see figure). The patients included in the study received a full vaccination course with 1 of the 3 US-licensed vaccines. Of note, most of these patients could be considered long-term survivors, as their cellular therapies were given a median of 25 to 30 months earlier for HCT recipients (n = 116) and 6 to 24 months earlier for CAR-T recipients (n = 14). However, in patients vaccinated within 6 months of therapy (n = 19), seroconversions rates were very disappointing: 50%, 37%, and 0% for autologous, allogeneic and CAR-T recipients respectively, supporting the current American Society of Hematology (ASH), American Society for Transplantation and Cellular Therapy (ASTCT), and European Society for Blood and Marrow Transplantation (EBMT) recommendations that time be allowed for immunological reconstitution before attempting vaccination. Finally, Dhakal et al highlight the use of prednisone and low immunoglobulin G levels as factors associated with the lack of antibody response in patients who undergo allogeneic HCT. These results are more reassuring than the 40% immune response rates in solid organ transplant recipients and are in line with previous reports suggesting 73% to 78% seroconversion rates in HCT recipients and 0% to 36% in CAR T-cell recipients after completing standard vaccination schedules.3-6 Unfortunately, the low patient numbers preclude a more granular analysis of factors associated with better immune response, and the study did not address safety. Two very recent publications have reported cases of graft-versus-host disease (GVHD), either presenting or worsening after vaccination with messenger RNA (mRNA) vaccines.5,7
Future studies are needed to evaluate how antibody type, quantity, and durability correlate with protection from contracting and transmitting SARS-CoV-2 for both healthy and immunosuppressed individuals. Other markers of immune response, such as T-cell response, have also been shown to be present alongside and/or independent of antibody formation.5,8 These immune responses should be further studied in cellular therapy recipients. It is unclear which vaccination platform will be most effective in immunosuppressed patients, although preliminary evidence suggests higher antibody titers after mRNA vaccines, compared with those obtained with adenovirus-based vaccines.3,9 Important issues such as optimal timing after initial cellular therapy and the impact of immune reconstitution, concomitant maintenance, and immunosuppressive therapy still must be elucidated. Prospective trials in allogeneic HCT recipients are urgently needed to address safety and the possible risk of GVHD exacerbation after vaccination. Finally, future studies are needed to evaluate the added value of an additional vaccine dose in patients lacking response after completing the current standard vaccination schedule, as suggested by promising results in solid organ transplant recipients who receive a third mRNA vaccine injection.6,10
By their description of the COVID-19 vaccination response in cellular therapy recipients, Dhakal and colleagues have generated data to support current international recommendations (https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients and https://www.ebmt.org/covid-19-and-bmt). They raise our awareness that more than one third of these patients may not develop antibody responses and additional strategies are needed to protect these fragile patients from COVID-19, even long after cellular therapy.
It will take more effort to build strong bridges over the quicksand, but every step counts. Dhakal and colleagues have helped us move forward.
Conflict-of-interest disclosure: H.S. reports personal fees and nonfinancial support from Incyte; grants, personal fees, and nonfinancial support from Novartis and BHS (Belgian Hematological Society); personal fees from, Janssen, Jazz Pharmaceuticals, and Takeda; and nonfinancial support from Gilead, the EBMT (European Society for Blood and Marrow transplantation), and the CIBMTR (Center for International Bone Marrow Transplantation Research) outside the submitted work. P.L. reports grants from SciLife Laboratory/KAW Foundation during the conduct of the study; grants and personal fees from MSD; and personal fees from AiCuris, Takeda, Pfizer, BMS, and OctaPharma, outside the submitted work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal