In this issue of Blood, Tsaknakis and colleagues1 used targeted next-generation sequencing (NGS) to determine the frequency and clinical significance of clonal hematopoiesis (CH) in a cohort of 185 patients with chronic idiopathic neutropenia (CIN).
CH is a common, aging-related phenomenon involving the expansion of hematopoietic stem cells with somatic mutations. The prevalence of CH varies with the detection method and variant allele frequency (VAF) threshold for defining clonality. That said, >10% of healthy individuals will have CH (defined as a VAF of ≥2%) by the age of 80 years.2,3 Importantly, although the presence of CH confers a significantly increased risk of the subsequent development of a hematologic malignancy, the overall incidence of such malignancies among individuals with CH is low.2,3 Thus, an understanding of the factors associated with the existence and evolution of CH is crucial to the identification of individuals who may warrant more vigilant monitoring.
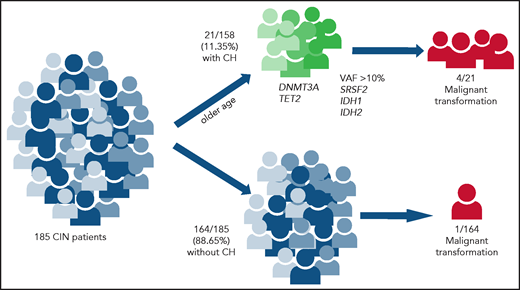
CH and progression to myeloid malignancy in CIN. Patients with CIN (n = 185) were assessed for CH by using targeted NGS. CH was detected in 21 (11.35%;) of them. Progression to myeloid malignancy was associated with VAF >10% and mutations in SRSF2 or IDH1/2. Created with BioRender.com.
CH and progression to myeloid malignancy in CIN. Patients with CIN (n = 185) were assessed for CH by using targeted NGS. CH was detected in 21 (11.35%;) of them. Progression to myeloid malignancy was associated with VAF >10% and mutations in SRSF2 or IDH1/2. Created with BioRender.com.
Chronic idiopathic neutropenia (CIN) is defined as isolated neutropenia without a known underlying congenital or acquired cause. CIN occurs in late childhood or adulthood, affects primarily women, and typically runs a benign course. CIN, also known as idiopathic cytopenia of undetermined significance-CIN (ICUS-N), is a subtype of ICUS. ICUS is characterized by persistent cytopenias of ≥1 blood lineages without meeting criteria for a myelodysplastic syndrome (MDS) or having other known etiologies.4 In previous studies, CH was detected in more than one-third of patients with ICUS (in which case it is known as clonal cytopenia of undetermined significance (CCUS) and was highly predictive of the subsequent development of a myeloid malignancy.5,6 The frequency of patients with isolated neutropenia (ICUS-N/CIN) in these prior studies of ICUS/CCUS was low, however, and therefore the specific incidence and prognosis of CH in these particular patients is not clear.
Tsaknakis and colleagues examined the frequency and clinical significance of CH in 185 patients with CIN. Using targeted NGS of genes recurrently mutated in myeloid malignancies, they found 21 of 185 (11.35%) with CH (defined as VAF ≥2%) and 164 of 185 (88.65%) without. Those with CH were significantly older than non-CH patients and had lower platelet counts. Notably, the presence of CH was not associated with the severity of neutropenia. Most patients (18 of 21) with CH had only 1 mutation, whereas 2 patients had 2 mutations each and 1 patient had 3, for a total of 25 somatic mutations in the 21 patients. Consistent with studies of CH in the general population, the most frequently mutated genes were DNMT3A and TET2.
Over the course of the study (median follow-up, 132 months), 5 patients with CIN developed myeloid malignancies (4 from the CH group and 1 from the non-CH group), demonstrating a significantly elevated risk (relative risk, 31.24) of transformation in the presence of CH. The VAFs of the mutant clones were >10% in all of the cases that transformed, and the most frequently mutated genes in those cases were SRSF2 and IDH1/2. In fact, all patients with SRSF2 and IDH1/2 mutations developed MDS or leukemia. Finally, 9 patients with CH had serial sampling for NGS. Of those 9 patients, 3 transformed into myeloid malignancy, and transformation was preceded by expansion of the variant clone. Notably, predictors of transformation in this study were similar to those previously reported in CH, which include the presence of multiple mutations, VAF >10%, the presence of mutations in specific high-risk genes (including TP53, IDH1/2, RUNX1, PHF6, and spliceosome genes), and altered red blood cell indices.2,7,8 Also consistent with previous data,7 isolated mutations in DNMT3A or TET2, although common among the patients with CH, were not predictive of transformation over the duration of this study.
Together, the new data show that, although CH confers an increased risk of malignancy in patients with CIN, the overall frequency of CH was much lower in this specific population (11%) than in prior studies of the broader ICUS population (>30%). The overall incidence of transformation was also lower in patients with CIN than in all patients with ICUS (2.7% vs 25%).5 Thus, this study highlights the unique nature of CIN as a subset of ICUS with a more favorable clinical course. Nevertheless, although much lower than the prevalence in the whole ICUS population, the relative prevalence of CH in patients with CIN who are <70 years of age was significantly higher than in age-matched individuals from the general population based on historical studies (relative prevalence, 2.56).2,3,9 Aside from age, clinical features, such as degree of neutropenia and other hematopoietic parameters (except for platelet counts, which were lower in the CH group but still within normal range and possibly explained by their older age), were not associated with CH. Therefore, CH should be considered in all patients with CIN. There are few specific recommendations for monitoring of CH in these patients; however, Tsaknakis et al noted that they follow up on patients with clonal CIN by using serial peripheral blood (PB) analysis and NGS and perform bone marrow analyses for concerning changes in PB counts or morphology. This approach seems reasonable, and the finding of an elevated (>10%) or increasing VAF or the presence of high-risk mutations should prompt concern for possible transformation.
The reasons for the reduced CH and transformation in CIN compared with other ICUS variants are not clear. Many gaps remain in the understanding of the pathogenesis of ICUS, as well as the development and progression of CH. Recent studies suggest that various hematopoietic stressors such as genotoxic stress, ribosome biogenesis stress, and inflammation can promote the development and progression of CH.10 Whether some of the same stressors contribute to cytopenias in ICUS remains to be determined. Further studies are clearly needed to elucidate the intrinsic and extrinsic drivers of both ICUS and CH and to identify the mechanisms by which specific mutations and environmental factors interplay to suppress normal hematopoiesis and to promote the expansion and/or transformation of mutant hematopoietic stem cells. Ultimately, better mechanistic insight will inform the identification and treatment of patients with cytopenia who are at risk for CH and malignant transformation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal