TO THE EDITOR:
Cancer immune evasion is a major hurdle for effective anticancer therapy. Key strategies of tumor cells to avoid T-cell recognition include downregulation of major histocompatibility complex (MHC) molecules and activation of immune checkpoints.1,2 Particularly, the immune checkpoint protein programmed cell death 1 (PD-1) and its ligands, PD-L1 and -L2, play a key role in repressing T-cell activity in the tumor microenvironment, not only in solid cancers but also in hematological malignancies. Specifically in advanced classic Hodgkin lymphoma (cHL), blockade of the PD-1/PD-L1/L2 axis yields outstanding clinical responses.3 Most cHLs are infiltrated by PD-1+ T cells, and the tumor cells show strong expression of PD-L1/PD-L2 related to copy-number alterations (CNAs) at chromosome 9p24.1, containing the loci for PD-L1/PD-L2 and JAK2,4 or alternatively, driven by Epstein-Barr virus (EBV) infection.5
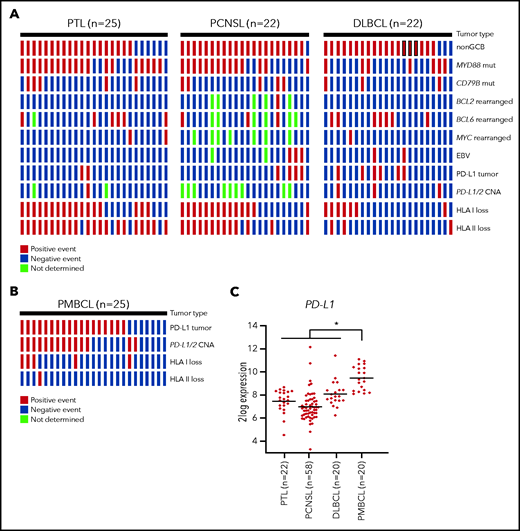
Primary testicular lymphomas (PTLs) and primary central nervous system lymphomas (PCNSLs) are uncommon and aggressive large-B cell lymphomas with a poor response to therapies and prognosis and shared molecular characteristics. PTLs and PCNSLs that arise at sites considered to be immune privileged6 display a high prevalence of activating somatic MYD88 mutations, often with a concurrent activating mutation in the immunoreceptor tyrosine–based activation motif of CD79B.7,8 Regarding immune evasion, PTLs and PCNSLs have been shown to exhibit frequent loss of HLA class I and II expression and/or loss of HLA loci.9,10 More recently, Chapuy et al reported frequent 9p24.1/PD-L1/2 CNAs and translocations with concomitant protein overexpression in PTLs and PCNSLs.11 A subsequent study by the same authors in a small series (n = 5) of patients with a PTL or PCNSL suggested clinical activity of PD-1 blockade with nivolumab.12 Based on these reports, several clinical trials exploring the efficacy of PD-1/PD-L1 blockade have been initiated (registered on www.clinicaltrials.gov as NCT02779101, NCT03255018, NCT04401774, and NCT02857426). In the current study, we revisited the immune evasion mechanisms operating in PTLs and PCNSLs. We confirmed a high frequency of HLA class I and II expression loss. However, with the exception of EBV+ PCNSLs, which were PD-L1+, PTLs and PCNSLs seldom expressed PD-L1 and, accordingly, 9p24.1/PD-L1/2 CNAs were rarely found.
We assessed HLA class I and II expression, PD-L1 expression, and 9p24.1/PD-L1/2 CNAs in a panel of lymphomas diagnosed as PTLs (n = 25) or PCNSLs (n = 22) according to the World Health Organization classification,13 using immunohistochemistry and fluorescence in situ hybridization (FISH), respectively (Table 1). For comparison, a set of diffuse large B-cell lymphomas (DLBCLs; n = 22), enriched for cases with a non–germinal center B-cell (GCB) phenotype to better match the PTLs and PCNSLs, were studied. All lymphomas were classified as either GCB- or non-GCB–like, using the immunohistochemical algorithm of Hans et al.14 In addition, the presence of somatic mutations in MYD88 and CD79B; of translocations of cMYC, BCL2, and BCL6; and of EBV status were assessed, as described previously.7,8 Consistent with previous reports,7,8,11MYD88 mutations were detected at high frequency in PTLs (76%) and PCNSLs (64%), and coexistent mutations in CD79B were often found. The presence of these MYD88 and CD79 mutations was almost mutually exclusive with translocations of cMYC, BCL2, and BCL6 or with EBV expression (Figure 1A). Apropos of immune evasion, we observed a high prevalence of loss of HLA class I expression in PTLs (68%) and PCNSLs (64%). Similarly, HLA class II loss was common in PTLs (84%) and PCNSLs (59%). These findings are in line with previous studies by Riemersma et al10 and Booman et al,15 who also reported frequent HLA class I and II loss in PTLs and PCNSLs. In marked contrast to the study by Chapuy et al,11 however, we barely detected expression of PD-L1 or 9p24.1/PD-L1/2 CNAs in our cohort of PTLs and PCNSLs. A notable exception were EBV+ tumors, which showed strong PD-L1 expression (Figure 1A). In the entire group of EBV− PCNSLs and PTLs (n = 42), only 3 cases with PD-L1 expression and 1 case with a 9p24.1/PD-L1/2 copy number gain were found. The frequency of PD-L1 expression and 9p24.1/PD-L1/2 CNA did not significantly differ from that in the DLBCLs. In approximately half of the cases, we observed variable, mostly weak, PD-L1 expression by macrophages in the tumor microenvironment. Representative PD-L1/PAX5 immunohistochemistry images are shown in supplemental Figure 1. All tissue samples were obtained during standard diagnostic procedures at the Academic Medical Center Amsterdam and affiliated hospitals and the University Medical Center Groningen in accordance with the local institutional board requirements.
Clinical and molecular characteristics of PTLs, PCNSLs and DLBCLs
| . | . | PTL . | PCNSL . | DLBCL . |
|---|---|---|---|---|
| (n = 25) . | (n = 22) . | (n = 22) . | ||
| Age, y | Median (range) | 74 (58-89) | 69 (49-83) | 63 (4-89) |
| Gender | Male | 100 (25/25) | 67 (14/21) | 64 (14/22) |
| Female | 0 (0/25) | 33 (7/21) | 36 (8/22) | |
| EBV status | Positive | 0 (0/25) | 15 (3/20) | 9 (2/22) |
| MYC | Rearranged | 8 (2/25) | 0 (0/15) | 5 (1/22) |
| BCL2 | Rearranged | 0 (0/25) | 6 (1/17) | 0 (0/22) |
| BCL6 | Rearranged | 25 (6/24) | 19 (3/16) | 32 (7/22) |
| MYD88 | Mutated | 76 (19/25) | 64 (14/22) | 32 (7/22)** |
| CD79B | Mutated | 20 (5/25) | 23 (5/22) | 14 (3/22) |
| COO | Non-GCB | 76 (19/25) | 95 (21/22) | 86 (19/22) |
| GCB | 24 (6/25) | 5 (1/22) | 14 (3/22) | |
| PD-L1 tumor | Negative | 92 (23/25) | 82 (18/22) | 73 (16/22) |
| Weak | 0 (0/25) | 4 (1/22) | 9 (2/22) | |
| Positive | 8 (2/25) | 14 (3/22) | 18 (4/22) | |
| PD-L1/2 CNA | Gain | 4 (1/23) | 0 (0/13) | 14 (3/22) |
| Polysomy | 0 (0/23) | 0 (0/13) | 0 (0/22) | |
| HLA class I | Loss | 68 (17/25) | 64 (14/22) | 32 (7/22)* |
| HLA class II | Loss | 84 (21/25) | 59 (13/22) | 14 (3/22)** |
| . | . | PTL . | PCNSL . | DLBCL . |
|---|---|---|---|---|
| (n = 25) . | (n = 22) . | (n = 22) . | ||
| Age, y | Median (range) | 74 (58-89) | 69 (49-83) | 63 (4-89) |
| Gender | Male | 100 (25/25) | 67 (14/21) | 64 (14/22) |
| Female | 0 (0/25) | 33 (7/21) | 36 (8/22) | |
| EBV status | Positive | 0 (0/25) | 15 (3/20) | 9 (2/22) |
| MYC | Rearranged | 8 (2/25) | 0 (0/15) | 5 (1/22) |
| BCL2 | Rearranged | 0 (0/25) | 6 (1/17) | 0 (0/22) |
| BCL6 | Rearranged | 25 (6/24) | 19 (3/16) | 32 (7/22) |
| MYD88 | Mutated | 76 (19/25) | 64 (14/22) | 32 (7/22)** |
| CD79B | Mutated | 20 (5/25) | 23 (5/22) | 14 (3/22) |
| COO | Non-GCB | 76 (19/25) | 95 (21/22) | 86 (19/22) |
| GCB | 24 (6/25) | 5 (1/22) | 14 (3/22) | |
| PD-L1 tumor | Negative | 92 (23/25) | 82 (18/22) | 73 (16/22) |
| Weak | 0 (0/25) | 4 (1/22) | 9 (2/22) | |
| Positive | 8 (2/25) | 14 (3/22) | 18 (4/22) | |
| PD-L1/2 CNA | Gain | 4 (1/23) | 0 (0/13) | 14 (3/22) |
| Polysomy | 0 (0/23) | 0 (0/13) | 0 (0/22) | |
| HLA class I | Loss | 68 (17/25) | 64 (14/22) | 32 (7/22)* |
| HLA class II | Loss | 84 (21/25) | 59 (13/22) | 14 (3/22)** |
Unless stated otherwise, data are expressed as percentage of cases that were positive (positive cases/total cases examined). The correlation between the clinical and molecular characteristics among the different tumor types was examined using the χ2 test. P-values were 2-sided and P < .05 was considered statistically significant. *P < .05; **P < .01.
COO, cell of origin.
Oncoprint plot. Shown is the molecular analysis of the PTLs, PCNSLs, and DLBCLs (A) and PMBCLs (B). All tissue samples were obtained during standard diagnostic procedures. Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue using anti-HLA class I (clone HC10, Nordic-MUbio), anti-HLA-DP,DQ,DR (clone CR3/43, DAKO), anti-PD-L1 (clone 22C3, DAKO), anti-PAX5 (clone SP43, Cell Marque), anti-CD10 (clone 56C6, ThermoFisher), anti-MUM1 (clone MUM1p, DAKO), anti-BCL2 (clone 124, Dako), and anti-BCL6 (clone PG-B6p, Dako) on a Labvision Autostainer 480S (ThermoFisher). Samples were scored positive for PD-L1 when membranous staining was observed in at least 5% of the malignant cells. Expression of EBV was determined by EBV-encoded RNA in-situ hybridization (EBER) probes (Biogenex). FISH for detection of BCL2, BCL6, and cMYC breaks was performed using probes and a FISH accessory kit (Dako). FISH for detection of PD-L1/2 CNAs was performed with the ZytoLight CD274/PDCD1LG2/CEN 9 Dual Color Probe (ZytoVision). FISH slides were evaluated in the context of serial sections stained for PD-L1 and B-cell markers (CD20 and PAX5) to localize tumor infiltrates. Samples were scored as having 9p24.1 disomy, polysomy, copy gain, or amplification. The presence of 3 or 4 green signals was classified as gain and the presence of 5 or more green signals was classified as amplification. Testing for somatic MYD88 and CD79B mutations was performed with allele-specific polymerase chain reaction, as described previously.7 Sanger sequencing was used to verify the presence of a mutation. (C) PD-L1 mRNA expression analysis of publicly available microarray data sets derived from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (accession numbers GSE10524, GSE61578, GSE34771 and GSE87371). All microarray data sets were generated with Affymetrix Human Genome U133 Plus 2.0 Array, and data analysis was performed with the R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl). The horizontal line represents the median expression within each group. Differences among subtypes were tested by Kruskal-Wallis test with the post hoc Dunn’s test. *P < .05.
Oncoprint plot. Shown is the molecular analysis of the PTLs, PCNSLs, and DLBCLs (A) and PMBCLs (B). All tissue samples were obtained during standard diagnostic procedures. Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue using anti-HLA class I (clone HC10, Nordic-MUbio), anti-HLA-DP,DQ,DR (clone CR3/43, DAKO), anti-PD-L1 (clone 22C3, DAKO), anti-PAX5 (clone SP43, Cell Marque), anti-CD10 (clone 56C6, ThermoFisher), anti-MUM1 (clone MUM1p, DAKO), anti-BCL2 (clone 124, Dako), and anti-BCL6 (clone PG-B6p, Dako) on a Labvision Autostainer 480S (ThermoFisher). Samples were scored positive for PD-L1 when membranous staining was observed in at least 5% of the malignant cells. Expression of EBV was determined by EBV-encoded RNA in-situ hybridization (EBER) probes (Biogenex). FISH for detection of BCL2, BCL6, and cMYC breaks was performed using probes and a FISH accessory kit (Dako). FISH for detection of PD-L1/2 CNAs was performed with the ZytoLight CD274/PDCD1LG2/CEN 9 Dual Color Probe (ZytoVision). FISH slides were evaluated in the context of serial sections stained for PD-L1 and B-cell markers (CD20 and PAX5) to localize tumor infiltrates. Samples were scored as having 9p24.1 disomy, polysomy, copy gain, or amplification. The presence of 3 or 4 green signals was classified as gain and the presence of 5 or more green signals was classified as amplification. Testing for somatic MYD88 and CD79B mutations was performed with allele-specific polymerase chain reaction, as described previously.7 Sanger sequencing was used to verify the presence of a mutation. (C) PD-L1 mRNA expression analysis of publicly available microarray data sets derived from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (accession numbers GSE10524, GSE61578, GSE34771 and GSE87371). All microarray data sets were generated with Affymetrix Human Genome U133 Plus 2.0 Array, and data analysis was performed with the R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl). The horizontal line represents the median expression within each group. Differences among subtypes were tested by Kruskal-Wallis test with the post hoc Dunn’s test. *P < .05.
In view of the vast discrepancy between our finding and those of Chapuy et al,11 we sought to further reinforce our data. First, to validate the techniques used to detect PD-L1 expression and 9p24.1/PD-L1/2 CNAs, a series of primary mediastinal B-cell lymphomas (PMBCLs; n = 25) were studied. Like cHLs, these lymphomas frequently show genetic aberrations in chromosome 9p24.1 with consequent PD-L1 overexpression.4 Indeed, we detected strong PD-L1 expression in a large proportion (68%) of the PMBCLs (supplemental Table 1), whereas 9p24.1/PD-L1/2 CNAs were observed in more than half of the cases (Figure 1B). Notably, in contrast to PTLs and PCNSLs, loss of HLA class I and II expression was uncommon in these PMBCLs (supplemental Table 1). Second, we studied PD-L1 messenger RNA (mRNA) expression data extracted from publicly available databases. These mRNA data reveal enhanced expression of PD-L1 in PMBCLs, but not in PTLs and PCNSLs, a finding consistent with the lack of PD-L1 expression in our current data set (Figure 1C). Notably, in line with our results, in a study of PCNSLs recently reported by Sethi et al,16 PD-L1 expression and/or 9p24.1/PD-L1/2 CNAs was barely found, except in EBV+ tumors, which were PD-L1+. A possible explanation for the discordantly high prevalence of 9p24.1/PD-L1/2 CN gains reported by Chapuy et al11 is an incorrectly low threshold setting in the quantitative polymerase chain reaction assays used to detect CNAs in the large “extension cohort” of their study. This error in threshold setting could also explain the strikingly lower percentage of CNAs in the initial “discovery cohort,” which were identified by using high-density single nucleotide polymorphism arrays.
In summary, our study indicates that different large B-cell lymphoma subtypes use distinct immune evasion strategies: PTLs and PCNSLs seem to use the PD1/PD-L1/2 checkpoint rarely, but instead use the loss of MHC expression as a major mechanism of immune evasion; in PMBCLs PD-L1 overexpression, often caused by 9p24.1/PD-L1/2 CNAs, appears to play a major role. This finding suggests that patients with PBMCL, but not those with PTL or PCNSL, are likely to benefit from treatment with PD-1/PD-L1 immune checkpoint inhibitors. Indeed, although a preliminary clinical study suggested beneficial activity of nivolumab treatment,12 the results of the phase 2 trial of nivolumab in patients with recurrent or refractory PCNSL or PTL (registered on www.clinicaltrials.gov as NCT02857426), which was initiated based on these initial findings, are highly disappointing: the objective response rate (ORR) was only 6.4% in 47 analyzed patients. Similarly, nivolumab monotherapy resulted in an ORR of only 3% to 10% in patients with relapsed/refractory DLBCL.17 In contrast, interim analysis of the phase 2 KEYNOTE-170 study (www.clinicaltrials.gov, NCT02576990) showed an ORR of 45% in patients with relapsed/refractory PBMCL.18 Considering the ongoing clinical trials involving immune checkpoint inhibitors in patients with PCNSL or PTL (www.clinicaltrials.gov, NCT04421560, NCT03770416, and NCT04609046), we believe that a critical reappraisal of the prevalence of PD-L1 expression and 9p24.1 alterations in these lymphomas is of crucial importance.
Acknowledgments
The authors thank M.F.M. de Rooij for helpful contributions to the data presentation.
This study was supported by a grant from Lymph&Co (M.J.K., S.T.P.).
Authorship
Contribution: M.M. performed the experiments, analyzed the data, and wrote the manuscript; A.A. designed the research, supervised the study, and analyzed the data; W.K. performed the experiments and analyzed the data; E.J.M.S.-T. and M.E.C.M.O. performed the experiments; C.G.S., A.L.N., and P.M.K. provided samples from patients with lymphoma; M.J.K., M.S., and S.T.P. designed the research, supervised the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven T. Pals, Department of Pathology, Amsterdam University Medical Centers, loc. AMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: s.t.pals@amc.uva.nl.
Original data are available in response to e-mail requests to Steven T. Pals (s.t.pals@amc.uva.nl).
The online version of this article contains a data supplement.
REFERENCES
Author notes
M.S. and S.T.P. are joint senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal