In this issue of Blood, Willier et al explored the differences in immunotarget expression in pediatric (ped) acute myeloid leukemia (AML) compared with adult AML. They identified CD33 and CLEC12A (CLL1) as the best combination for immunotherapy in ped AML. Their findings underscore the need to delineate immunotarget expression levels prior to (pre-)clinical application of chimeric antigen receptor (CAR)–T-cell treatment.1
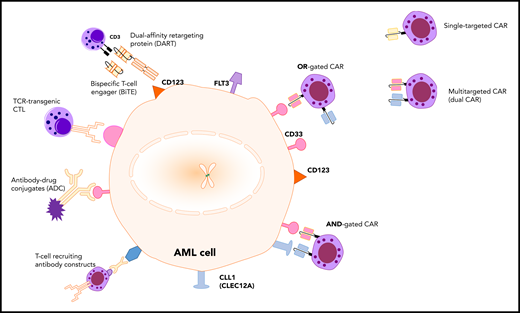
Immunotherapy targets and treatment modalities in AML. The AML cell surface markers, CLEC12A (CLL1), CD33, CD123, and FLT3, can theoretically be used as targets for various immunological modalities, such as antibody-drug conjugates (ADC), T-cell receptor (TCR)–transgenic cytotoxic T lymphocytes (CTL), T-cell recruiting antibody constructs, bispecific T-cell engagers (BiTE), and CAR-T cells. CAR-T cells can be designed using single-targeting CARs or multitargeted CARs, such as dual CARs.
Immunotherapy targets and treatment modalities in AML. The AML cell surface markers, CLEC12A (CLL1), CD33, CD123, and FLT3, can theoretically be used as targets for various immunological modalities, such as antibody-drug conjugates (ADC), T-cell receptor (TCR)–transgenic cytotoxic T lymphocytes (CTL), T-cell recruiting antibody constructs, bispecific T-cell engagers (BiTE), and CAR-T cells. CAR-T cells can be designed using single-targeting CARs or multitargeted CARs, such as dual CARs.
Ped AML is much less common than acute lymphoblastic leukemia (ALL) in children. Whereas the overall survival for ped AML currently reaches 75%, outcomes remain poor in patients with relapsed or refractory disease despite salvage therapy with intensive reinduction chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT).2 Following the clinically successful trials with CD19-directed CAR–T-cell treatment in childhood and young adult ALL,3 substantial efforts were made to duplicate this success in AML. Unlike B-cell malignancies, which exclusively express B-cell lineage antigens, cellular therapy in AML has been hampered by the lack of AML-specific antigens and by coexpression of myeloid antigens on normal hematopoietic stem cells and/or progenitor cells.4
Willier et al studied the heterogeneity of ped AML and have provided AML target antigen expression data from ped patients. The goal of this work was to define targets for immunotherapy in ped AML.
The authors analyzed sorted leukemic blasts from 36 bone marrow (BM) samples of ped AML using whole RNA-sequencing and flow cytometry. They compared the results with early hematopoietic precursor cells from healthy BM. Differential overexpression of a number of targets was found in AML blasts and a clustering of the ped AML samples by KMT2A and t(8;21) status. The highest flow cytometric expression (percentage of cells and median fluorescence intensity) on ped AML blasts together with lowest expression on healthy progenitors was found for the targets CD33 and CLEC12A. In addition, a high FLT3 surface expression was detected in KMT2A-mutated infant AML samples.
The authors calculated an estimate of on-target but off-leukemia toxicity based on proteome data and concluded that CD33 and CLEC12A had noninferior expression profile in healthy tissues compared with other target molecules. Hence, they concluded that the combination of targeting of CD33 and CLEC12A represented the most promising target for ped AML and CD33/FLT3 for KMT2A-mutated infant AML.
Previously, several immunotherapeutic treatment modalities (see figure) have been developed and tested in early phase trials. Mylotarg (Gemtuzumab Ozogamicin), which targets CD33 on the surface of AML blasts, was the first antibody-drug conjugate approved by the US Food and Drug Administration for ped AML.2,5 CD123, the α chain of the interleukin 3 receptor, is used as target for antibody-drug conjugates, BiTEs, dual-affinity retargeting agents (DARTs), and CARs. Phase 1 to 2 studies investigating the CD123 × CD3 bispecific DART Flotetuzumab (#NCT04158739) and CAR-T cells against CD123 (#NCT04318678, #NCT03766126, #NCT02159495) or CD33 (#NCT03971799, #NCT01864902) are currently recruiting ped AML patients. Another study evaluating multi–CAR-T cells that recognize specific targets, such as CD33, CD38, CD123, CD56, Muc1, and CLEC12A (#NCT03222674), is ongoing. In 2018, results of a phase 1 study with a “compound CAR-T” against CLEC12A and CD33 in refractory AML were presented.6
Target antigen selection for immunotherapy is challenging. However, in AML, the target antigen selection has been largely based on studies from adult patients. The study of Willier et al is unique, as it thoroughly checks target expression on RNA and cell surface level in ped AML, providing solid scientific ground for selecting suitable targets. Interestingly, the cell surface expression of CD123, which is broadly expressed on adult AML, was found in only a minority of samples in the ped AML. Based on these findings, the use of CD123 as immunotarget in ped AML should be discouraged as unlikely to be beneficial.
To increase target specificity and cover the heterogeneity of AML, antigen combinations for CAR-T therapy have been proposed in adult AML.7 Consequently, dual CAR-T cells, compound CAR-T cells, and even multi CAR-T cells have been created. Willier et al propose dual targeting, adapted to the age of the patients and to the AML genetic groups. Their suggested immunotargets in non-KMT2A mutated ped AML are CD33 plus CLEC12A, but the choice of employing CARs that simultaneously target both or either one of these antigens is left open. Nevertheless, this choice is crucial because it determines the coverage ratio, the specificity for AML cells, and the on-target but off-leukemia toxic effects. Whereas CD33 is widely expressed on AML blasts, it is also present on healthy hematopoietic cells. CLEC12A, on the other hand, has the advantage of high expression in AML and no expression on healthy hematopoietic cells, except for mature myeloid cells, such as monocytes.8 About 60% of AML samples with CD33 expression coexpress CLEC12A. Dual CAR-T cells directed against both antigens will cover 60% of the ped AML patients. Dual targeting designed against either one of the 2 targets will result in an increased coverage, but less specificity and profound myelosuppression. This prolonged myeloablation increases the risk of infection and transfusion dependence. It will also require HSCT rescue or interventions that limit CAR–T-cell persistence.
Ideally, an AML-specific antigen overexpressed on tumor cells, but absent in normal healthy tissues, should be used in ped AML immunotherapy. Unfortunately, no such antigen has been identified yet, although research projects are currently ongoing. For example, specific proteins, such as TCR ϒ chain alternate reading frame protein (TARP), are being used in the generation of TARP–T-cell receptor transgenic cytotoxic T cells and have shown in vitro cytotoxicity in AML cell lines and ped AML samples.9
The road from discovery of a target to introduction of an immunotherapeutic clinical trial is tough because of ped AML heterogeneity and the limited number of patients. Further success in developing targeted therapies for ped AML will come not only from international collaboration and collaborative clinical studies but also from a better understanding of specific disease mechanisms and characteristics. Preclinical testing, as in the paper of Willier et al, is therefore a valuable and indispensable step in this challenging journey.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal