Abstract
Adult T-cell leukemia/lymphoma (ATL) is a highly aggressive T-cell malignancy that arises in a proportion of individuals who are long-term carriers of human T-lymphotropic virus type 1. The median survival of aggressive subtypes is 8 to 10 months; with chemotherapy-based approaches, overall survival has remained largely unchanged in the ∼35 years since ATL was first described. Through the use of 4 representative case studies, we highlight advances in the biological understanding of ATL and the use of novel therapies such as mogamulizumab, as well as how they are best applied to different subtypes of ATL. We discuss the implementation of molecular methods that may guide diagnosis or treatment, although we accept that these are not universally available. In particular, we acknowledge discrepancies in treatment between different countries, reflecting current drug licensing and the difficulties in making treatment decisions in a rare disease, with limited high-quality clinical trial data.
Which HTLV-1 carriers develop ATL?
Adult T-cell leukemia/lymphoma (ATL) is a highly aggressive T-cell malignancy that typically follows decades of asymptomatic chronic human T-lymphotropic virus type 1 (HTLV-1) infection and usually arises in carriers who acquired HTLV-1 during childhood via breastfeeding. HTLV-1 is prevalent worldwide, with the highest known prevalence in Japan, the African continent, Caribbean islands, Melanesia, and South America.1 The median age of presentation in Japan is 60 to 70 years, but for reasons that are not understood, median age is significantly lower in patients presenting in the United States, Europe, and South and Central America (40-55 years). In Brazil, pediatric cases have been described.2-7
HTLV-1 integrates as a single proviral copy within each CD4+ host genome and each asymptomatic carrier carries >104 to 105 distinct HTLV-1–infected T-cell clones, one of which typically undergoes cellular transformation and monoclonal expansion and drives malignant disease.8,9 The exact mechanism by which HTLV-1 causes malignancy has not been fully elucidated, but chronic HTLV-1 infection is regarded as the necessary first hit in a multistep oncogenic process. The lifetime risk of developing ATL is 1% to 5% among all carriers, but this risk is virtually entirely within high–proviral load (PVL) asymptomatic carriers (PVL > 4%, proportion of infected mononuclear cells) whose lifetime risk is >20%.10-12 Targeted sequencing has identified premalignant clonal populations bearing known driver mutations in the peripheral blood of HTLV carriers up to 10 years prior to development of ATL, and these mutations increase in total number and variant allele frequency in the 6 months prior to clinical presentation, which may in the future enable earlier and more effective intervention to prevent the development of ATL.13,14
Case 1
A 48-year-old Jamaican man presents unwell with night sweats, weight loss, and confusion. Investigations revealed a minor lymphocytosis, hypercalcemia, and elevated lactate dehydrogenase (LDH; 2000 IU/mL); CT imaging showed widespread lymphadenopathy with bulky retroperitoneal lymph nodes (12 cm). Lymph node biopsy identified an atypical lymphoid infiltrate with immunohistochemical markers positive for CD2, CD3, CD4, CD5, B-cell lymphoma 2 (BCL2), MUM1, T-cell receptor β (TCR β), programmed cell death 1 (PD1), and CD25 (patchy) and negative for CD20, BCL5, CD10, CD21, CD23, CD30, and cyclin D1. Ki67 was >95%. The appearances were reported as consistent with peripheral T-cell lymphoma (PTCL), not otherwise specified. HTLV-1 serology was positive, and he was diagnosed with acute ATL with bulky lymphadenopathy. Further HTLV molecular workup demonstrated a monoclonal HTLV-1–infected T-cell population in the blood (HTLV-1–infected cells identified by flow cytometry as CD3+, CD4+, CCR4+, and CD26−, and 92% of infected cells expressed a single TCRvB8.1). He was treated with 4 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) plus high-dose methotrexate (MTX; 3 g/m2). During chemotherapy, he received opportunistic infection prophylaxis with cotrimoxazole, fluconazole, and aciclovir. Strongyloides serology was negative. After 4 cycles of chemotherapy, a positron emission tomography (PET)/CT scan confirmed a complete metabolic response, and he underwent a haploidentical stem cell transplant. He was commenced on the integrase inhibitor raltegravir at day 0 to prevent neoinfection of donor stem cells, to be continued for as long as the HTLV-1 PVL remained undetectable by quantitative polymerase chain reaction. The transplant course was relatively uneventful, other than some grade 1 skin graft-versus-host disease (GVHD) at day 60 requiring topical steroids only. The patient is now 9 months posthaploidentical transplant and remains well and in remission.
Diagnosis and staging of ATL, and limitations of Shimoyama/World Health Organization classification
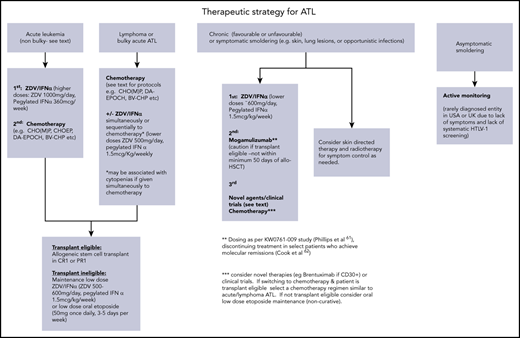
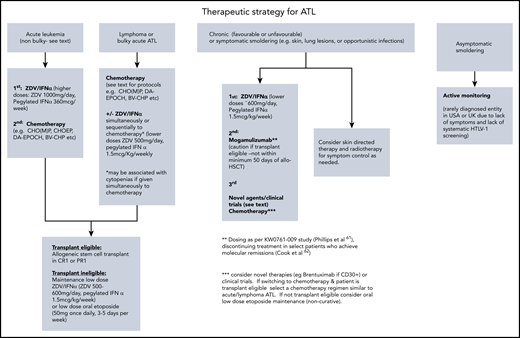
This patient was diagnosed with the acute subtype of ATL with bulky lymphadenopathy based upon lymphocytosis, hypercalcemia, LDH, and bulky lymph nodes identified on PET/CT imaging (classification detailed in Table 1). The original Shimoyama classification remains unchanged and is divided into 4 different clinical subtypes: 2 aggressive subtypes (acute and lymphoma) and 2 relatively indolent subtypes (chronic and smoldering).15 More recently, a spectrum of primary cutaneous ATL has been described, some of which are highly aggressive.16-18 The fact that the classification has not changed has been useful to standardize and compare clinical outcomes across countries and allows for a comparison of clinical data over decades, although this has not been done prospectively. However, there are some limitations to the classification: first, the criteria for lymphoma subtype includes <1% circulating abnormal lymphocytes. This means that patients with typical lymphoma presentations and normal blood counts but found to have >1% abnormal lymphocytes on a blood smear are classified as acute, alongside those with aggressive leukemic but minimally nodal disease. This has important treatment implications because patients with leukemic subtypes are much more likely to respond to mogamulizumab or zidovudine (ZDV) and interferon-α (IFN-α) treatment.19,20 Second, a diagnosis of smoldering ATL may be made based on the presence of >5% abnormal lymphocytes alone, but there is evidence that the percentage of abnormal lymphocytes correlates with PVL, and may be seen in the peripheral blood smear of those with nonmalignant infection, such as high-PVL asymptomatic carriers or HTLV-1 inflammatory disease.21
Diagnosis and classification of ATL
| Subtype . | Asymptomatic HTLV-1 carrier . | Smoldering . | Chronic . | Acute . | Lymphoma . |
|---|---|---|---|---|---|
| HTLV-1 serology | + | + | + | + | + |
| Monoclonal integration of provirus | − | + | + | + | + (lymph node) |
| Lymphocyte count | Normal | Normal | Elevated | Elevated | Normal |
| Percentage circulating abnormal lymphocytes | <5% | ≥5% or <5% if ATL skin and lung lesions present | ≥5% | ≥5% | ≤1% |
| Hypercalcemia | − | − | − | Normal or high | Normal or high |
| LDH | Normal | Normal or ≤1.5 ULN | Normal or <2 ULN | Normal or high | Normal or high |
| Skin or lung involvement | − | + | + | + | + |
| Bone marrow or splenic involvement | − | − | + | + | + |
| Bone, gastrointestinal, or CNS involvement | − | − | − | + | + |
| Subtype . | Asymptomatic HTLV-1 carrier . | Smoldering . | Chronic . | Acute . | Lymphoma . |
|---|---|---|---|---|---|
| HTLV-1 serology | + | + | + | + | + |
| Monoclonal integration of provirus | − | + | + | + | + (lymph node) |
| Lymphocyte count | Normal | Normal | Elevated | Elevated | Normal |
| Percentage circulating abnormal lymphocytes | <5% | ≥5% or <5% if ATL skin and lung lesions present | ≥5% | ≥5% | ≤1% |
| Hypercalcemia | − | − | − | Normal or high | Normal or high |
| LDH | Normal | Normal or ≤1.5 ULN | Normal or <2 ULN | Normal or high | Normal or high |
| Skin or lung involvement | − | + | + | + | + |
| Bone marrow or splenic involvement | − | − | + | + | + |
| Bone, gastrointestinal, or CNS involvement | − | − | − | + | + |
−, negative; +, positive; CNS, central nervous system; ULN, upper limit of normal.
The patient was staged with PET/CT imaging at baseline, which is our preference, because this is more likely to identify extranodal sites of disease; however, it does not currently influence patient management, so contrast-enhanced CT scanning remains a reasonable alternative.22-24 The 12-cm lymph node mass and high LDH would predict for a high risk of tumor lysis and we would manage patients according to local protocols, with IV fluids, allopurinol and rasburicase as indicated. There is no standard definition of bulk, but we would regard individual lymph nodes ≥3 cm as bulky or cases with smaller-sized lymphadenopathy but with multiple and widespread distribution. Bone marrow biopsy is generally not required in the diagnosis and staging of ATL.22
ATL may be difficult to distinguish from other PTCL subtypes based upon immunohistochemistry or flow cytometry alone. ATL cells have a characteristic morphology (“flower" cells) and a mature T-cell phenotype, with expression markers usually CD2, CD5, HLA-DR, and TCR αβ+ with aberrant loss of CD7 and CD3−/dim. ATL cells typically express CCR4 and CD25. CD25 is also expressed in Sezary syndrome and T-cell prolymphocytic leukemia. ATL cells are usually CD4+ and CD8−, although rare cases are CD4−/CD8+ or CD4+/CD8+. CD30 expression is variable (discussed in the "Chemotherapy for lymphoma or bulky disease" section). Given the variations in immunohistochemistry of the transformed T cells, HTLV-1 serology is essential and recommended in all cases of PTCL or cutaneous T-cell lymphoma.25 Identification of monoclonal viral integration by Southern blot or polymerase chain reaction (PCR)-based methods is also recommended during diagnostic workup.22,25 PVL assays are not widely available in the United States, and are not routinely indicated in the diagnosis of ATL but may be helpful in specific scenarios, for example, where there is histological uncertainty between lymphoma subtype ATL vs a PTCL in a HTLV-1 carrier: the PVL in peripheral blood of ATL cases is typically >4% and the median ratio of PVL in lymph node compared with blood is 5:1; thus, in the United Kingdom, we use this to clarify difficult diagnostic cases.12 The distinction between other PTCL subtypes and ATL is crucial because there may be differing treatment and transplantation strategies.
Patients are immunocompromised and opportunistic infections are common including, Pneumocystis jirovecii pneumonia, aspergillosis, candidiasis, strongyloidiasis, and cytomegalovirus infection.26 Strongyloides serology is recommended at diagnosis to ensure appropriate treatment prior to commencing therapy alongside prophylaxis against opportunistic infections with aciclovir, cotrimoxazole, and fluconazole. Hepatitis B coinfection is not uncommon in HTLV-1 carriers, and we would include prophylaxis against reactivation with tenofovir (or antivirals as per local practice). Although there is no clear evidence to support the use of raltegravir during transplantation, it is my practice in the United Kingdom to incorporate within transplant protocols (from day 0, continued as long as PVL remains undetectable), to minimize infection of donor stem cells from reservoirs of HTLV-1–infected cells.
Chemotherapy for lymphoma or bulky disease
Clinical trials specifically in ATL are rare, but wherever possible patients should be recruited into trials of biologically plausible agents for similar clinical presentations. For example, PTCL studies may allow lymphoma type ATL or CTCL/Sezary studies may include smoldering or chronic ATL. This patient was diagnosed with aggressive bulky acute ATL, which requires chemotherapy-based approaches, and if clinically suitable, followed by allogeneic stem cell transplantation (alloSCT). Although response rates to induction treatment may be relatively high (60% to 70%), relapse is inevitable and transplantation offers a chance of cure. Combination chemotherapy alone has been of limited success, possibly due to the intrinsic resistance of ATL cells as well as to the associated immunosuppression and the frequent poor performance status of patients.
Since the 1980s, clinical trials conducted in Japan have investigated the feasibility and efficacy of both single-agent chemotherapy agents (such as pentostatin and cladribine) and combination chemotherapy.27,28 Combination regimens are generally associated with an increased response rate (∼60%), however, response duration and overall survival (OS) remain short (usually <1 year) and there are very few long-term survivors. Chemotherapy combinations vary geographically. In Japan, the vincristine, cyclophosphamide, doxorubicin, and AMP, prednisone (VCAP)–doxorubicin, ranimustine, and prednisolone (AMP)–vindesine, etoposide, carboplatin, and prednisolone (VECP) protocol has been widely adopted.
This followed the randomized trial from the Japan Clinical Oncology Group (JCOG) 9801 study that showed an improved response rate in younger patients (<56 years) for intensified combined treatment with VCAP/AMP/VECP compared with CHOP-14 alone (cyclophosphamide, vincristine, doxorubicin, and prednisolone). The complete response (CR) rate including CR unconfirmed was 40% in VCAP-AMP-VECP vs 25% in CHOP-14 (P = .02) with a trend toward improved 3-year survival (24% vs 13%; P = .085, and median survival time (MST) was 12.7 vs 10.9 months.29 However, in the VCAP/AMP/VECP arm the toxicities were significant compared with CHOP alone (grade 4 neutropenia, grade 4 thrombocytopenia, and grade 3 or 4 infection were 98% v 83%, 74% v 17%, and 32% v 15%, respectively) including 3 treatment-related deaths in in the intensive arm. We do not use this intensified regimen because it is associated with increased toxicities and is not curative. Some agents (vindesine and ranimustine) are not available outside of Japan and so our preference is for CHOP- or etoposide-based regimens such as etoposide, cyclophosphamide, vincristine, doxorubicin and prednisone (CHOEP), dose-adjusted etoposide, cyclophosphamide, vincristine, doxorubicin, and prednisone (DA-EPOCH); cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternated with high-dose cytarabine and MTX (hyper-CVAD); ifosfamide, etoposide, and cytarabine (IVAC). Malpica et al reported a modified VCAP/AMP/VECP regimen, omitting ranimustine and vincristine in place of vindesine.6 There is no evidence that any of these regimens are superior to one another.
Mogamulizumab is a monoclonal antibody that targets CCR4 expressed in neoplastic cells in ∼90% of ATL cases and mutated in 26%. This expression has been associated with cutaneous manifestations and poor prognosis.30 Mogamulizumab has been approved in Japan for newly diagnosed aggressive ATL in combination with VCAP-AMP-VECP chemotherapy on the basis of higher response rates compared with VCAP-AMP-VECP chemotherapy alone (52% vs 33%). CR rates varied among target lesions: rates were high in peripheral blood, intermediate in skin, and low in lymph nodes.31 Longer-term follow-up showed no significant difference in median PFS or OS between the 2 arms, and 3-year OS was 45% and 50% in the VCAP-AMP-VECP plus mogamulizumab and VCAP-AMP-VECP arms, respectively.32 Notably, an increased risk of steroid-refractory acute GVHD was observed when used pretransplantation and so we do not use mogamulizumab in the front-line setting (reviewed Fuji and Shindo33 ).
Brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisolone (CHP) has recently been included in current National Comprehensive Cancer Network (NCCN) guidelines for front-line treatment of CD30+ PTCL including ATL. CD30 expression on HTLV-1–infected cells correlates with clonal disease progression (median CD30 expression on CD4+, CD25+, CCR4+ cells is 1.58% in healthy carriers, 15% chronic ATL, 36% acute ATL), and correlates with both the expression of soluble interleukin 2 (IL2) receptor, and with the presence of polylobated “flower” cells,34 although its significance in clonal selection or disease evolution is not understood. The NCCN’s recommendation is based on the ECHELON-2 study,35 which included 7 patients with ATL (of 452 trial participants). The median PFS of all trial participants was 48.2 months in the BV plus CHP arm and 20.8 months in the CHOP group (P = .01) but there were no subgroup analysis of ATL patients due to too few patients. There are too little clinical data to draw meaningful conclusions about the efficacy of BV in ATL. However, where it is available (United States), we would support its use in front-line setting until more real-world data are available.
CNS prophylaxis
At least 10% to 20% of patients with aggressive ATL experience central nervous system (CNS) disease, with 50% of acute relapses occurring in the CNS. CNS prophylaxis has been incorporated into the sequential JCOG clinical trials and is recommended as a standard of care for acute or lymphoma subtypes treated with either chemotherapy or ZDV/IFN-α. There are no randomized studies in ATL designed specifically to determine which prophylactic strategies reduce CNS events, and most of the relevant data comes from subset analyses of sequential JCOG studies, confounded by varying treatment protocols, timing, dosing, and chemotherapy agents used. A recent international consensus report suggests that asymptomatic patients with aggressive ATL should have a diagnostic lumbar puncture/intrathecal (IT) chemotherapy at the end of the first chemotherapy or equivalent ZDV/IFN-α upon successful disease control (detailed further in “Case 2”).25
However, there is much controversy in high-grade BCLs with regards to the benefits of IT vs effective systemic therapy which crosses the blood-brain barrier, which may overcome the limitations of IT therapy and achieve even and predictable concentrations throughout both the leptomeningeal and parenchymal compartments, although this has not been studied specifically in ATL. My preference (L.B.C.) in the United Kingdom is to incorporate systemic high-dose MTX (3 g/m2) into standard CHOP given on day 10 of a 21-day cycle, aiming for 6 cycles, and reserve IT prophylaxis for cases in which systemic treatment is contraindicated or difficult to give. In this clinical case, the haploidentical transplant protocol also contained busulfan and thiotepa, which should provide further CNS protection. By contrast, in the United States, A.A.P. routinely uses dose-adjusted etoposide, doxorubicin, vincristine, prednisolone, cyclophosphamide (EPOCH) or, more recently, BV-CHP for up to 6 cycles with cerebrospinal fluid (CSF) sampling at diagnosis or with cycle 2, adding IT MTX prophylaxis (day 1).
For patients presenting with CNS disease concurrent to systemic ATL, we treat with either intensified cyclophosphamide, cytarabine, doxorubicin, vincristine, MTX (CODOX M) ifosfamide, etoposide, and cytarabine (IVAC) protocol with intrathecal MTX and cytarabine on days 6 and 17 or the hyperCVAD regimen (cyclophosphamide, vincristine, doxorubicin, dexamethasone, alternating with systemic MTX, cytarabine, and with intrathecal MTX, cytarabine).
Allogeneic transplantation
The greatest experience of allogeneic HSCT (allo-HSCT) in ATL is in Japan, where over 2000 patients have been registered, compared with the relatively rare cases reaching transplant elsewhere. Utilizing myeloablative conditioning protocols, the transplant-related mortality (TRM) has been high, whereas the TRM rates of patients who received reduced-intensity stem cell transplantation is decreased. Retrospective data suggests that ATL transplant survival is improved by early transplant (<100 days since diagnosis) and while in a CR.36 The European Group for Blood and Marrow Transplantation’s (EBMT) Lymphoma Working Party has confirmed alloSCT may also salvage ATL patients.37 Outside of Japan, however, it can be difficult to find suitably matched donors since the majority of cases arise in ethnic minorities not reflective of transplant donor registries, with family members often living overseas, and with the added complication of HTLV-1–infected siblings.38 If the only available donor is HTLV-1+, we would assess the donor for clonal expansions (PVL and an assay for HTLV-1 clonal expansion39 ) and if the donor is low risk we would proceed. The feasibility of haploidentical transplants and a “donor for everyone,” offers great hope for patients with ATL although currently remains experimental.
In summary, this patient was treated with CHOP combined with high-dose MTX because intensified protocols such as VCAP-AMP-VECP or those incorporating monoclonal antibodies mogamulizumab or brentuximab have not been shown to be curative but associated with increased toxicities. These monoclonals are also not approved in the United Kingdom. There is no evidence that a fixed number of chemotherapy cycles should be given prior to allogeneic transplant, and the patient was transplanted as soon as remission was confirmed by PET/CT, and the donor available. Common regimens and practical aspects of use are detailed in Table 2 and a suggested treatment algorithm for each ATL subtype (Figure 1).
Regimens in ATL
| Study/year . | Regimen . | Evaluable patients, n . | ORR, % . | Survival . | Notes . |
|---|---|---|---|---|---|
| Marçais et al40 /2020 | Retrospective arsenic trioxide with ZDV/ IFN consolidation following induction with chemotherapy or ZDV/IFN | 9 | Median OS 28 mo from start of consolidation (range, 1-70 mo) | As2O3 administered for median 6 mo (2-24 mo) | |
| Phillips et al41 /2019 | Randomized phase 2 mogamulizumab vs investigator’s choice of chemotherapy Relapsed/refractory | 71 | 0 15, investigator assessment | N/A | 96% positive for CCR4, most benefit in leukemic ATL |
| Sharma et al42 /2017 | Phase 2 alemtuzumab Relapsed/refractory | 29 | 52 | 6 mo | Most benefit seen in leukemic disease, all patients reactivated CMV |
| Ishida et al43 /2016 | Multicenter phase 2, lenalidomide Relapsed/refractory | 26 | 42 | OS, 20.3 mo; PFS, 4 mo | Surprisingly long OS given short PFS; possibly more indolent relapses |
| Ratner et al44 /2015 | Phase 1/2 EPOCH with bortezomib and raltegravir front line | 18 | 67 | 6.2 mo | No increased benefit with addition of bortezomib or raltegravir |
| Ishitsuka et al45 /2015 | Phase 2 bortezomib Relapsed/refractory | 15 | 7 | N/A | Study terminated due to lack of efficacy |
| Ishida et al31,32 /2015; update 2019 | Randomized phase 2 VCAP/AMP/VECP ± mogamulizumab front line | 54 | 86 (+moga) 75 (−moga) | 3-y OS, 45% (+moga arm) vs 50% (−moga) | Improved CR rate with moga (52% vs 33%) No increased PFS/OS, increased risk GVHD Study led to Japanese approval |
| Ishida et al19 /2012 | Multicenter phase 2 mogamulizumab in relapsed refractory | 28 | 50 | 14 mo | Study led to Japanese approval in relapsed refractory disease |
| Ceesay et al 46 /2012 | Phase 2 study CHOP + anti-CD25 monoclonal front line | 15 | 60 | 10 mo | No benefit to addition of anti-CD25 |
| Bazarbachi et al20 /2010 | Meta-analysis ZDV/IFN in aggressive ATL | 254 (n = 75 frontline) | 66 | (5 y) 23% | Retrospective, lymphoma patients better outcomes with chemotherapy Acute and chronic leukemics benefit most from ZDV/IFN p53 mutations resistant |
| Hodson et al47 /2011 | Retrospective study ZDV/IFN at any point for acute or lymphoma | 73 | HR, 0.23 reduced risk of death | Retrospective | |
| Ratner et al; AIDS Malignancy Consortium48 / 2009 | Phase 2 EPOCH followed by ZDV/IFN Front line | 19 | 58 | mPFS and mOS 6 mo | Prospective phase 2 study ZDV/IFN maintenance did not add to survival |
| Kchour et al49 /2009 | Phase 2 ZDV/IFN/ As2O3 newly diagnosed symptomatic chronic ATL | 10 | 100 (7 CR, 2 vgPR,* 1 PR) | 30 d arsenic combined with ZDV/IFN followed by ZDV/IFN. Median study follow up 8 mo (range, 2-15 mo), all patients alive, none progressed. | |
| Tsukasaki et al29 /2007 | Randomized phase 3 VCAP/AMP/VECP vs CHOP14 front line | 118 | 72 66 | (3 y) 24% 13% | More toxicities, only 32% completed planned treatment |
| Hermine et al50 /2004 | Phase 2 IFN/arsenic trioxide Relapsed/refractory | 7 | 7.5 mo | Treatment discontinued after median 22 d due to toxicity (n = 3) or progression (n = 4) |
| Study/year . | Regimen . | Evaluable patients, n . | ORR, % . | Survival . | Notes . |
|---|---|---|---|---|---|
| Marçais et al40 /2020 | Retrospective arsenic trioxide with ZDV/ IFN consolidation following induction with chemotherapy or ZDV/IFN | 9 | Median OS 28 mo from start of consolidation (range, 1-70 mo) | As2O3 administered for median 6 mo (2-24 mo) | |
| Phillips et al41 /2019 | Randomized phase 2 mogamulizumab vs investigator’s choice of chemotherapy Relapsed/refractory | 71 | 0 15, investigator assessment | N/A | 96% positive for CCR4, most benefit in leukemic ATL |
| Sharma et al42 /2017 | Phase 2 alemtuzumab Relapsed/refractory | 29 | 52 | 6 mo | Most benefit seen in leukemic disease, all patients reactivated CMV |
| Ishida et al43 /2016 | Multicenter phase 2, lenalidomide Relapsed/refractory | 26 | 42 | OS, 20.3 mo; PFS, 4 mo | Surprisingly long OS given short PFS; possibly more indolent relapses |
| Ratner et al44 /2015 | Phase 1/2 EPOCH with bortezomib and raltegravir front line | 18 | 67 | 6.2 mo | No increased benefit with addition of bortezomib or raltegravir |
| Ishitsuka et al45 /2015 | Phase 2 bortezomib Relapsed/refractory | 15 | 7 | N/A | Study terminated due to lack of efficacy |
| Ishida et al31,32 /2015; update 2019 | Randomized phase 2 VCAP/AMP/VECP ± mogamulizumab front line | 54 | 86 (+moga) 75 (−moga) | 3-y OS, 45% (+moga arm) vs 50% (−moga) | Improved CR rate with moga (52% vs 33%) No increased PFS/OS, increased risk GVHD Study led to Japanese approval |
| Ishida et al19 /2012 | Multicenter phase 2 mogamulizumab in relapsed refractory | 28 | 50 | 14 mo | Study led to Japanese approval in relapsed refractory disease |
| Ceesay et al 46 /2012 | Phase 2 study CHOP + anti-CD25 monoclonal front line | 15 | 60 | 10 mo | No benefit to addition of anti-CD25 |
| Bazarbachi et al20 /2010 | Meta-analysis ZDV/IFN in aggressive ATL | 254 (n = 75 frontline) | 66 | (5 y) 23% | Retrospective, lymphoma patients better outcomes with chemotherapy Acute and chronic leukemics benefit most from ZDV/IFN p53 mutations resistant |
| Hodson et al47 /2011 | Retrospective study ZDV/IFN at any point for acute or lymphoma | 73 | HR, 0.23 reduced risk of death | Retrospective | |
| Ratner et al; AIDS Malignancy Consortium48 / 2009 | Phase 2 EPOCH followed by ZDV/IFN Front line | 19 | 58 | mPFS and mOS 6 mo | Prospective phase 2 study ZDV/IFN maintenance did not add to survival |
| Kchour et al49 /2009 | Phase 2 ZDV/IFN/ As2O3 newly diagnosed symptomatic chronic ATL | 10 | 100 (7 CR, 2 vgPR,* 1 PR) | 30 d arsenic combined with ZDV/IFN followed by ZDV/IFN. Median study follow up 8 mo (range, 2-15 mo), all patients alive, none progressed. | |
| Tsukasaki et al29 /2007 | Randomized phase 3 VCAP/AMP/VECP vs CHOP14 front line | 118 | 72 66 | (3 y) 24% 13% | More toxicities, only 32% completed planned treatment |
| Hermine et al50 /2004 | Phase 2 IFN/arsenic trioxide Relapsed/refractory | 7 | 7.5 mo | Treatment discontinued after median 22 d due to toxicity (n = 3) or progression (n = 4) |
CMV, cytomegalovirus; mOS, median OS; mPFS, median PFS; N/A, not applicable; ORR, overall response rate; PR, partial response.
vgPR indicates very good partial response, defined as normalization of the complete blood count, and disappearance of all measurable tumors for at least 1 month, but persistence of >5% atypical lymphocytes on the peripheral blood smear.
Case 2
A 67-year-old Haitian man presented with fever, night sweats, and bulky cervical adenopathy. Initial laboratory results were notable for atypical lymphocytosis, hypercalcemia, an elevated LDH and peripheral flow cytometry identified an abnormal T-cell population expressing CD3, CD4, CD25, negative for CD7, CD8, TCR γ/δ, CD57, CD56, CD30, and CD16. HTLV serology was positive. PET/CT confirmed a nodal conglomerate neck mass (15 cm with standard uptake value of 14.9) with further fluorodeoxyglucose (FDG)-avid lymphadenopathy and soft tissue deposits. Lymph node biopsy showed a diffuse infiltration of the tissue by an atypical lymphoid population with a similar immunophenotype identified in the blood.
He was diagnosed with bulky acute subtype ATL and similar to the first case, received combination chemotherapy with 1 cycle of dose-adjusted EPOCH, but was switched to hyperCVAD when routine CSF sampling after cycle 1 revealed asymptomatic leptomeningeal disease. He received antimicrobial prophylaxis throughout and continued to complete 4 cycles of hyperCVAD chemotherapy. His CSF cleared and he was referred for alloSCT after cycle 2 of induction chemotherapy and a 10 of 10 matched unrelated donor was identified. During pretransplant assessments, a repeat PET/CT showed resolution of all lymphadenopathy but new cavitating lung lesions. A CT-guided lung biopsy was nondiagnostic but surgical lung biopsy grew Aspergillus sp. He was treated with AmBisome and later switched to isavuconazole.
He became quite debilitated and his Hematopoietic Cell Transplantation–Specific Comorbidity Index (HCT-CI) score was 7 conferring a 40% risk of nonrelapse mortality.51,52 After multidisciplinary discussion, he was deemed ineligible for transplant.
He was started on maintenance ZDV/IFNα, which was continued for 16 months. At a routine clinic visit he was noted to be newly pancytopenic, concerning for relapse. A bone marrow biopsy revealed a hypercellular marrow with an abnormal blast population expressing dim CD45, CD117, dim CD33, bright CD36, bright CD71, CD4, CD35, CD105 and CD42a, and CD61 (partial) and negative for cCD3, CD19, CD10, CD13, CD34, HLA-DR, TdT, and myeloperoxidase. In addition, an abnormal CD3+, bright CD2+, CD5+, CD7−, CD4+, CD25+ T-cell population was identified (1% of analyzed cells). The findings were consistent with acute myeloid leukemia, with concurrent involvement by the patient’s previously diagnosed ATL. He was treated with azacitidine and venetoclax but died in hospice of progressive AML 4 months later; his ATL remained in remission, 28 months after original diagnosis.
Strategies for non–transplant-eligible patients
The outcome of patients with aggressive subtypes who are not fit enough for allogeneic transplant is poor, with only a few long-term survivors from chemotherapy or ZDV/IFN-α–based treatments alone.
Assessing response in ATL can be challenging given the different disease compartments involved. The current response criteria are based on normalization/reduction in the size of enlarged lymph nodes and extranodal sites of disease, as well as a decrease in the involvement of peripheral blood, bone marrow, and skin.22 Despite a complete response to induction chemotherapy by PET/CT scan and early referral to a transplant unit, he became transplant ineligible. Biopsy of his lung process was paramount to establishing a diagnosis as the differential of opportunistic infection, malignancy, or other inflammatory process was quite broad, despite receiving appropriate antimicrobial prophylaxis. Pulmonary disorders have been associated with HTLV-1 infection, with indigenous Australians and a UK cohort showing a high prevalence of bronchiectasis.53,54
Once his fungal pneumonia was adequately treated, he was started on maintenance therapy. For patients not proceeding to alloSCT, we review the risks, benefits, and alternatives to maintenance therapy and try to encourage all patients to participate on a clinical trial if available or start maintenance ZDV/IFN-α despite few studies demonstrating its potential role. In a retrospective British cohort of 73 patients (29 acute; 44 lymphoma), ZDV/IFNα was studied concurrently and sequential to first-line therapy for aggressive ATL and the use of ZDV/IFNα at any time prolonged survival and was the sole factor associated with reduction in risk of death (hazard ratio [HR], 0.23; 95% confidence interval [CI], 0.09-0.06; P = .002).47 In a small prospective US study using dose-adjusted EPOCH with maintenance ZDV/IFN-α (n = 19), patients showed an overall response rate (ORR) of 58%, with 2 complete remissions (CRs); however, the maintenance therapy did not seem to add to the efficacy.48 A single case reported low-dose lenalidomide maintained a CR in a patient with aggressive ATL for 24 months.55 In a retrospective study, long-term oral maintenance combination chemotherapy with vincristine or MTX, prednisolone, etoposide and cyclophosphamide (OPECMPEC) or with daily oral etoposide and prednisolone improved quality of life.56 The role of novel agents such as mogamulizumab or brentuximab in the maintenance of transplant ineligible patients is yet to be explored.
The precise dose of ZDV/IFNα maintenance is unknown and doses between 500 mg and 1000 mg daily in divided doses are used as tolerated with IFNα (pegylated IFNα-2a) 1.5 μg/kg weekly (rounded upwards to nearest vial), approximately equivalent to 3 million units 3 days per week.6,47 If not well tolerated, we switch patients to etoposide 50 mg once daily, given for 3 to 5 days per week until disease progression. Because this is not a curative approach, any follow-up imaging may be guided by clinician judgement.
In summary, for transplant-ineligible aggressive subtypes with nodal disease, induction chemotherapy followed by maintenance ZDV/IFN-α or oral low-dose chemotherapy is our standard approach.
Case 3: chronic ATL
A 54-year-old Iranian woman was found to have an isolated lymphocytosis following a routine blood count. The rest of her laboratory tests were unremarkable other than an elevated LDH 400 IU/mL (upper limit, 243 IU/mL). Blood film morphology showed pleomorphic lymphocytes, some with clefted nuclei, and occasional flower cells. Flow cytometry showed a dominant population of T cells expressing CD3+, CD4+, CD25+, CD30+ (20%), CD7−, and HTLV serology was positive. Extended investigations revealed an HTLV-1 PVL of 70% and a further flow cytometry confirmed the ATL population were HTLV-1 infected (CD4+, CCR4+, CD26−) and expressed a dominant TCRvB. CT imaging was unremarkable. She was diagnosed with chronic ATL and treated with ZDV/IFN-α, achieved a hematological remission within a few weeks, followed by a molecular response a few months later (PVL reduced to 5%). She experiences some ongoing side effects reducing compliance, mostly fatigue, but continues at present.
Active monitoring vs upfront treatment of indolent ATL?
Chronic ATL is not an indolent disease, and a high LDH, high blood urea nitrogen, and low albumin levels have been identified as poor prognostic factors. The presence of ≥1 of these factors is regarded as chronic aggressive or unfavorable.22 A Brazilian study, in which patients with indolent ATL were followed for a maximum duration of 14 years, reported that the MST of chronic and smoldering types were 18 months and 58 months, respectively, and the OS rates were <20% in both types.57 This poor prognosis was confirmed in a single-center study in Japan of 90 patients with indolent ATL that reported a MST of 4.1 years, and the estimated 15-year OS rates were 14.1% with no plateau in the survival curve. In that study, 65.1% of patients died of acute ATL with a median time to transformation of 18.8 months.57 Taken together, where ZDV/IFN-α is available and would be tolerated, there is no longer a role for active monitoring in chronic subtypes of ATL.
Therapy with ZDV/IFN-α
Several antiretrovirals used for HIV have proven efficacy against HTLV-1 in tissue culture, including reverse transcriptase inhibitors ZDV and tenofovir, and the integrase inhibitor raltegravir.58,59 Whether ZDV/IFN-α work through an antiviral effect remains controversial with arguments in favor of either a direct cytotoxic effect or an antiviral effect: HTLV-1 encodes 2 oncogenic factors Tax and HTLV-1 basic leucine zipper factor (HBZ), both of which play critical roles in cellular proliferation, and there is strong evidence that the quality of the host response to these 2 antigens plays a critical role in determining the risk of HTLV-1–associated diseases.60 Tax, encoded by sense messenger RNA, activates NF-κB, cyclic AMP response element binding (CREB), activator protein 1 (AP-1), and nuclear factor of activated T cells (NF-AT), among others, upregulating various host genes related to cell activation and proliferation. HBZ is encoded by the antisense HTLV-1 genome and activates transforming growth factor–β/Smad pathway, promoting FOXP3 expression, but suppresses CREB, AP-1, NF-AT, and classical NF-κB pathways, competing with Tax functions.61,62 Several studies have however shown minimal or absent viral gene expression in peripheral blood of infected carriers, with the exception of the antisense gene HBZ. However, HTLV-1 gene expression in vivo is not silent since most infected individuals maintain antibodies to HTLV-1 structural proteins with frequent detectable Tax and HBZ-specific cytotoxic T lymphocytes. Kinpara et al reported that IFN-α inhibited viral gene expression and induced cell-cycle arrest, and when combined with ZDV triggered P53 signaling and apoptosis in these cells.63 By contrast, a report demonstrated long-term treatment with ZDV/IFN-α significantly inhibited HTLV-1 reverse transcriptase activity in responding ATL patients but not in resistant patients.64 Because reverse transcriptase-mediated viral replication does not occur in the malignant cells these results suggest that any antiviral effect occurs in the HTLV-1 infected nonmalignant cells, which may play a major role in supporting the survival of ATL cells (reviewed in Journo and Mahieux65 ).
It is also unclear whether reactivation of HTLV-1 expression from either malignant or non-malignant cells after induction therapy may contribute to treatment resistance. A chemotherapy trial that included raltegravir, did not show a survival benefit, suggesting that either raltegravir did not completely inhibit viral replication in vivo, or that viral replication does not contribute to chemotherapy resistance.48 By contrast, a report demonstrated long-term treatment with ZDV/IFN-α significantly inhibited HTLV-1 reverse transcriptase activity in responding ATL patients but not in resistant patients.64 These results suggest that any antiviral effect occurs in the HTLV-1–infected nonmalignant cells, which may play a major role in supporting the survival of ATL cells.
A number of phase 2 studies investigated the ZDV/IFN-α combination in patients with ATL, including those who had failed prior cytotoxic chemotherapy.66-68 For the leukemia subgroup of patients, in particular, these results are superior to any chemotherapy regimen.20 A multinational retrospective meta-analysis on 254 patients confirmed that response rate and survival are improved when these drugs are used as first-line therapy, particularly in leukemic subtypes. Of all subtypes, those who were treated with ZDV/IFN-α therapy alone had a major survival advantage with a 5-year OS at 46% for patients who received antiviral therapy alone (n = 75) compared with 20% for those receiving chemotherapy alone (n = 75) and 12% (n = 55) for those receiving chemotherapy followed by ZDV/IFN-α maintenance. Patients with acute, chronic, and smoldering ATL significantly benefited from first-line antiviral therapy, whereas those patients with ATL lymphoma experienced a better outcome with chemotherapy. In acute ATL, achievement of complete remission with ZDV/IFN-α therapy resulted in 82% 5-year OS and doses as high as ZDV 1.5 g twice daily given intravenously with IFN-α 5 million to 10 million units (MU) twice daily have been reported. Responding patients can be dose reduced as an outpatient to ZDV 600 mg twice daily and IFN-α 5 MU once or twice daily. Patients with prolonged clinical responses can be tapered down to maintenance doses (eg, ZDV 300 mg twice daily and IFN-α 3 MU 3 times week).6 ZDV/IFNα in chronic and smoldering ATL resulted in 100% 5-year OS and multivariate analysis confirmed that first-line ZDV/IFN-α therapy significantly improves OS of patients with ATL (hazard ratio 0.47, 95% CI 0.27- 0.83, P = .021).20 A prospective study of ZDV/IFN-α vs active monitoring in indolent ATL is under way in Japan (trial JCOG1111C).
One of the major challenges of ZDV/IFN-α treatment are interferon side effects resulting in poor compliance, particularly for patients who may require indefinite treatment. Our local practice is to treat all patients with chronic subtype (regardless of prognostic characteristics) and those with symptomatic smoldering ATL with ZDV/IFN-α indefinitely, but it may be discontinued in select patients that achieve a deep molecular response by PVL and clonality measures (eg, TCRvB or clone-specific integration site quantification), but to stop treatment requires close follow-up to reinitiate treatment if measurable disease occurs.69 Responding patients with chronic/smoldering ATL maintained on ZDV/IFN-α do not require allogeneic transplantation in first remission.
Mogamulizumab has been investigated in clinical trials as a monotherapy in relapsed refractory aggressive ATL, including chronic subtypes. In a Japanese multicenter phase 2 study, treatment with mogamulizumab resulted in a 50% response rate and median OS of 13.7 months in 28 patients with relapsed or refractory aggressive ATL and in an international randomized phase 2 study of mogamulizumab vs investigator’s choice of pralatrexate, gemcitabine plus oxaliplatin, or dexamethasone, cisplatin, and cytarabine (DHAP) showed an ORR of 15% with mogamulizumab compared with only 0% in the investigator’s choice chemotherapy arm.19,41 Mogamulizumab responses are more effective in leukemic rather than those with nodal disease, and mogamulizumab in chronic aggressive ATL may result in durable clinical and molecular remission.70 In particular, activating mutations of CCR4 are associated with superior responses to mogamulizumab.30 At present mogamulizumab monotherapy (without chemotherapy) has not been used in upfront treatment of smoldering or chronic subtypes of ATL, but it is likely to be a focus of future studies.
In transplant-eligible patients, mogamulizumab should not be used within 50 days of transplant because of the increased reports of acute GVHD.71-73 Mogamulizumab is also associated with grade 2-4 drug hypersensitivity skin eruptions in up to ∼50% individuals, although there is some evidence that those with grade 2 rashes might have better progression-free survival (PFS).74 This is suggested due to mogamulizumab depleting T-regulatory subsets that may stimulate antitumor activity. However, the use of steroids to treat grade 2-4 skin rashes may offset any antitumor benefit, increase the infection risk, and Stevens Johnson, or toxic epidermal necrolysis may be directly fatal.
In summary, this patient with chronic unfavorable ATL was treated with ZDV/IFN and will continue providing she responds, and the therapy is reasonably tolerated. In the event of intolerance or progressive disease, we would switch to mogamulizumab monotherapy, keeping chemotherapy and allogeneic transplantation as a backup plan, also ensuring a minimum mogamulizumab washout period of 50 days to avoid complications of mogamulizumab associated acute GVHD.
Case 4
A 51 Haitian woman presented with a hyperpigmented rash on her upper extremities and trunk. A punch biopsy revealed angiocentric and epidermotropic CD25+ and CD4+ T-cell lymphoma. HTLV-1 serology was positive and PCR detected the HTLV-1 genome in her skin biopsy. Her blood counts were normal and a PET/CT scanning revealed no FDG-avid disease. Peripheral blood flow cytometry revealed a minor population of abnormal CD2+, CD3+, CD4+, CD5+, CD25+ T cells with discrete loss of CD7 compose (3% of the analyzed cells). She was diagnosed with symptomatic smoldering ATL.
She was initially offered ZDV/IFN-α therapy, which she declined, and was instead treated with phototherapy. Her rash progressed after 4 months and she was started on ZDV/IFN-α. She was initially started on zidovudine 300 mg twice daily and IFN-α 3 millions units 3 times weekly with a goal to titrate to higher doses, however, she was not able to tolerate higher doses of therapy; she managed for 1 year with some noncompliance due to fatigue. She then developed adenopathy and constitutional symptoms. A PET/CT scan revealed new FDG-avid cutaneous lesions and a left axillary lymph node. Repeat skin biopsy showed a large T-cell lymphomatous transformation. She was recruited into a clinical trial and treated with both lenalidomide and romidepsin (NCT02232516). Biopsy-proven progression occurred after 6 months and she was switched to chemotherapy, receiving a cycle each of gemcitabine, CHOP, dexamethasone, oxaliplatin, cytarabine (DHAX), and mogamulizumab but continued to progress. She also received palliative radiotherapy to a preauricular node. She entered hospice 4 years from initial diagnosis.
Symptomatic smoldering ATL is not an indolent disorder, and in a large Japanese retrospective study of ATL with cutaneous lesions (where ZDV/IFN is not available), 5-year survival rate was 0% in nodulotumoral and erythrodermic types compared with more than 40% in multipapular, plaque, and patch types. More recently a newer entity “primary cutaneous tumoral” (PCT-ATL) has been described, without leukemic or lymph node involvement. PCT-ATL is distinct, with cutaneous lesions appearing as tumors that grow rapidly and whose histology shows large, atypical cells with a high proliferative index.25 PCT-ATL frequently has a progressive and fatal clinical course that resembles aggressive ATL and we would treat with higher doses ZDV/IFNα and close monitoring, reserving chemotherapy and allo-HSCT for nonresponders. Skin directed therapies such as phototherapy provide localized disease control which may be useful in patients affected by pruritus, and scaling, or where systemic therapies such as ZDV/IFN are not tolerated.75,76
Novel therapies in ATL
Patients with relapsed ATL should participate on prospective clinical trials and new insights into molecular biology have provided ideas for future studies. Exome sequencing has revealed frequent mutated genes involved in pathways signaling downstream of the T-cell receptor, activation of NF-κB including recurrent gain of function mutations in PLCG1 (36%), PRKCB (33%), CARD11 (24%), VAV1 (18%), in T-cell trafficking receptors CCR4 (29%) and CCR7 (11%), and gene-fusions (CTLA4-CD28 and ICOS-CD28) . In addition, molecules associated with immune surveillance such has HLA-A/B and FAS are recurrently affected, epigenetic regulators such as RhoA and TET2 frequently mutated, as well as genes involved with immune surveillance leading to PD ligand 1 overexpression.77-80 Inhibitors of these pathway mediators could be useful novel therapy although, a trial including bortezomib, an NF-κB inhibitor, did not provide significant benefit when added to induction chemotherapy or in relapsed/refractory setting.44,45
Therapy available for relapsed/refractory PTCL has been used for ATL in the United States, including the antifolate agent pralatrexate, histone deacetylase (HDAC) inhibitors belinostat and romidepsin. In the development of pralatrexate, there were a total of 3 patients included on the pivotal phase 1 and 2 trials however in a retrospective study from New York City, the ORR was 19% and the risk of Stevens-Johnson syndrome may be higher in ATL patients compared with PTCL.81,82 In the phase 2 Southwest Oncology Group trial of alisertib, an aurora kinase inhibitor, 4 patients with ATL were treated, and 1 of them responded.83 There are no data from prospective clinical trials on the use of the use of HDAC inhibitors (belinostat and romidepsin), but in a small case series of patients with relapsed or refractory ATL, romidepsin resulted in modest response rates and was associated with a higher rate of cytopenias.84
In Japan, a phase 2 study of single-agent lenalidomide showed a 42% ORR in relapsed or refractory ATL. Interestingly, although the median PFS with this approach was 4 months, the median OS was 20 months.43 The most frequent grade ≥3 adverse events were hematologic, which were all manageable and reversible. Lenalidomide was not effective in 4 patients treated with ATL in the United States in a study that closed early due to poor patient accrual.85
Aberrant PD ligand 1 expression through 3′ untranslated region changes were also found in 27% ATL samples which suggest that immune checkpoint therapy could have a role in ATL treatment79 although a report of hyperacute progression in 3 patients treated with nivolumab in ATL lead to early closure of the US study.86
Several other agents have been explored in very small numbers of patients: arsenic trioxide (AS2O3) in combination with IFN-α has been shown to selectively kill HTLV-I–infected cells, through reversion of the constitutive activation of NF-κB and degradation of the Tax oncoprotein by the proteasome87 and was first investigated in relapsed/refractory aggressive ATL (n = 7), with modest effect and some toxicities50 and later investigated in a front-line symptomatic chronic ATL study which showed 100% ORR, but reported only a median 8 months follow up.49 A recent retrospective report of AS203/ZDV/IFN-α given as consolidation therapy following induction treatment in aggressive ATL reported a median OS at 28 months from initiation of consolidation, which suggests this may be an option for transplant ineligible patients, although requires a significant number of hospital visits and monitoring.40 Alemtuzumab may also have some activity in leukemic ATL: a phase 2 study of demonstrated responses in 12 of 15 patients with acute ATL and 2 of 3 patients with chronic ATL whereas responses were seen in only 1 of 11 patients with lymphoma but the duration of response was short (median duration 1.4 months for whole group, compared with 14.5 months for responders). CMV reactivation was observed in all patients.42 We would not advocate use of alemtuzumab in lymphoma subtypes outside of clinical trials where it is currently being explored in combination with novel agents.
There is a paucity of data regarding the efficacy of radiation therapy in patients with ATL. A retrospective analysis of 10 consecutive patients with relapsed or refractory ATL treated with radiation therapy (mean, 35.4 Gy; range, 12-60 Gy) reported that all patients had an at least partial response with 40% attaining a CR within the treatment field.88 Toxicity was generally mild to moderate and may provide palliation of patients who have symptoms related to a single disease site.
A variety of novel agents are currently under investigation or development in early phase studies including inhibitors of Enhancer of zeste homolog 2 (EZH2) and EZH1,89-91 cobormarsen an inhibitor of mir-155 (NCT02580552), belinostat in combination with AZT/IFN (NCT02737046), recombinant interleukin-15 with mogamulizumab (NCT04185220), brentuximab with chemotherapy (NCT03264131), ruxolitinib (NCT01712659). An anti-ATL therapeutic vaccine, consisting of Tax peptide-pulsed dendritic cells, induced Tax-specific CTL responses in ATL patients and exhibited favorable clinical outcomes, unless Tax-defective ATL clones emerged.92 Viral and tumor antigens (such as Tax, CCR4) provide targets for development of chimeric antigen receptor T–cell programs in ATL.
In summary, this patient had relapsed refractory disease where skin-directed treatment and radiotherapy were used for symptom control. We would consider this patient group for available clinical trials of novel agents.
Conclusions
ATL is currently managed according to clinical subtype, but is a heterogeneous disease without truly effective therapies and with relatively few patients reaching allogeneic transplant. The genomic landscape of ATL is being elucidated, with further biological understanding, and the development of novel agents, an era beyond the Shimoyama classification is on the horizon, where we should aim to deliver tailored therapies. There is an urgent need for clinical trials in ATL that also incorporate molecular knowledge to understand and improve outcomes. This will require international collaboration.
Acknowledgments
L.B.C. was supported, in part, by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC). A.A.P. was supported, in part, by the Harold Amos Medical Faculty Career Development Program (of the Robert Wood Johnson Foundation and American Society of Hematology).
Authorship
Contribution: L.B.C. and A.A.P. designed research, contributed case studies, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lucy B. Cook, Catherine Lewis Centre, Hammersmith Hospital, Imperial College London, Du Cane Rd, London, W12 0HN United Kingdom; e-mail: l.cook@imperial.ac.uk.