Key Points
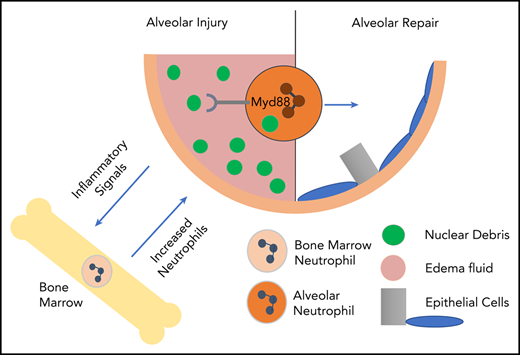
Neutrophils acquire the ability to engulf and breakdown extracellular DNA at sites of inflammation, which helps with optimal repair.
DNase I is able to rescue the phenotype of DNA-debris accumulation in neutropenic mice exposed to acid-induced injury.
Abstract
Neutrophils are critical mediators of host defense in pathogen-induced and sterile inflammation. Excessive neutrophil activation has been associated with increased host pathology through collateral organ damage. The beneficial aspects of neutrophil activation, particularly in sterile inflammation, are less well defined. We observed accumulation of nuclear debris in the lungs of neutropenic mice exposed to acid-induced injury compared with wild type. Size analysis of DNA debris showed that neutropenic mice were unable to degrade extracellular DNA fragments. In addition, we found that neutrophils are able to differentially express DNA-degrading and repair-associated genes and proteins. Once neutrophils are at sites of lung inflammation, they are able to phagocytose and degrade extracellular DNA. This neutrophil-dependent DNA degradation occurs in a MyD88-dependent pathway. The increased DNA debris in neutropenic mice was associated with dysregulated alveolar repair and the phenotype is rescued by intratracheal administration of DNase I. Thus, we show a novel mechanism as part of the inflammatory response, in which neutrophils engulf and degrade extracellular DNA fragments and allow for optimal organ repair.
Introduction
Neutrophil recruitment to sites of sterile injury is commonly observed, but is of unknown significance. Data from a number of groups suggest that sterile inflammatory responses are deleterious and perpetuate pathology.1-3 Other studies show that the integrated response to sterile injury removes inciting materials and facilitates repair.4 Although sterile lesions exhibit commonalities with infection,5 resolution of sterile injury remains poorly understood.
We previously demonstrated that neutrophil accumulation in the lung after acid aspiration is necessary for repair.6 Mechanisms by which neutrophils facilitate lung repair are lacking, though in models of sterile liver injury, neutrophils7,8 are necessary for debris clearance, which precedes repair. In these systems, neutrophils have been shown to take up small DNA particles that presumably allowed for improved revascularization.7 In models of mechanical lung injury, neutrophils and neutrophil-derived matrix metalloproteinase-9 were shown to be critical for epithelial and matrix repair.9 Similarly, in the central nervous system, clearance of myelin debris is essential for repair and remyelination.10-12
We show extensive debris accumulation in sterile lung injury in neutrophil-depleted mice and hypothesize that neutrophil clearance of DNA-containing debris is critical for repair. Identification of DNase-containing infiltrating neutrophils suggests that DNA clearance is specifically targeted. These neutrophils are able to degrade and internalize DNA in vitro, through an MyD88-dependent pathway. Interestingly, intratracheal DNase I supplementation enhanced lesion repair in neutropenic mice, suggesting a mechanism in lung repair.
Study design
Mice
Mice were housed in specific pathogen-free conditions at the Children’s Hospital of Philadelphia (CHOP) animal facility. Protocols were approved by the CHOP Institutional Animal Care and Use Committee. GCSF−/−-C57BL/6J (002398) and wild-type (WT)-C57BL/6J mice (The Jackson Laboratory) were used. Sedated mice lungs were injured with 0.1 N HCl as described.6,13
Histology
Lungs were fixed under constant pressure with 10% formaldehyde, dehydrated with ethanol washes, embedded in paraffin, and sectioned at 5 μm. Hematoxylin-and-eosin staining was used as previously described.6
Immunohistochemistry
Following deparaffinization, samples were treated with antigen-unmasking solution (Vector Laboratories). Immunohistochemistry antibodies included: podoplanin clone 8.1.1 (University of Iowa), prosurfactant protein C (EMD Millipore), and Ki67 (Abcam). The TSA biotin staining kit (Perkin-Elmer) was used to prevent cross-reactivity.
ELISA
Bronchoalveolar lavage (BAL) and cell counts were analyzed as previously described.14 BAL and serum testing were performed with enzyme-linked immunosorbent assay (ELISA) kits: mouse granulocyte colony-stimulating factor (G-CSF; R&D Systems) and immunoglobulin M (IgM; eBioscience). Protein quantitation was performed by BCA protein assay (ThermoScientific).
Lung digestion and flow cytometry
Single-cell suspensions were generated by chopping lung, enzymatic digestion in 480 U/mL collagenase I (Life Technologies), 50 U/mL dispase (Collaborative Research), and 0.33 U/mL DNase I (Roche) and passage through 100-μM and 40-μM cell strainers (BD Falcon) following 30-minute incubation. Red blood cells were lysed with ACK solution and cells were resuspended in fluorescence-activated cell sorting buffer. Fixation and permeabilization were conducted per protocol (FOXP3 kit; eBioscience). Antibodies for fluorescence-activated cell sorting (Accuri-C6) included: Ki67-allophycocyanin (BioLegend) and prosurfactant protein C (EMD Millipore) conjugated to DyLight488 (Abcam). Data were analyzed on CFlow-Plus (BD Biosciences).
Analysis of BAL DNA
DNA content was analyzed by Nanodrop and by Bioanalyzer.
Mass spectrometry
One milliliter of BAL was albumin and IgG depleted (Sigma-Aldrich) per protocol. Following trichloroacetic acid precipitation, samples were run on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel, Coomassie stained, excised in 3 segments, destained with 50% methanol/1.25% acetic acid, reduced with 5 mM dithiothreitol, alkylated with 20 mM iodoacetamide, washed with 20 mM ammonium bicarbonate, and dehydrated with acetonitrile. Gel was incubated overnight at 37°C with trypsin. Peptides were extracted with 0.3% trifluoroacetic acid followed by 50% acetonitrile. Tryptic digests were analyzed by liquid chromatography with tandem mass spectrometry (MS/MS) on a QExactive HF mass spectrometer (ThermoFisher). Peptides were separated by reversed-phase high-performance liquid chromatography and eluted at 300 nL/min. The mass spectrometer repetitively scanned m/z from 300 to 1400 (R = 240 000) followed by data-dependent MS/MS scans on the 20 most abundant ions.
Protein data
Sequences were aligned with SEQUEST and were analyzed in Scaffold. Differential expressions were analyzed using Ingenuity Pathway Analysis (Qiagen). Proteins >2 standard deviations from identity were considered differentially expressed. Peptides were analyzed by sample and quantity. Significance was defined as peptides present in at least 2 samples.
Statistics
Means were compared using the Student t test or 2-way analysis of variance. P values <.05 were considered significant.
Results and discussion
G-CSF−/− neutropenia highlights failure of debris clearance
Absence of G-CSF is associated with severe neutropenia.15,16 BAL content of acid-induced lung injury in G-CSF−/− mice had significantly reduced neutrophils,6 and tissue specimens 24 hours after injury had an 81.1% reduction in neutrophils (P = .036) compared with WT.6 BAL-resident macrophage numbers were not significantly altered in G-CSF−/− mice compared with WT mice at baseline and after lung injury (supplemental Figure 1, available on the Blood Web site). Acid-injured lung tissue from G-CSF−/− mice also had significant alveolar debris compared with WT mice (Figure 1A).
Neutropenic mice have more DNA-containing debris following acid injury of the lung. (A) Lungs from injured areas of WT and G-CSF−/− lungs were fixed 24 hours after unilateral acid-induced lung injury and stained with hematoxylin-and-eosin sections. Scale bar, 100 μm. The extent of debris is apparent. (B) BAL fluid sampled 24 hours after acid-induced lung injury from neutropenic (G-CSF−/−) mice and WT mice were compared using mass spectroscopy after depleting albumin and IgG. We appreciated a marked increase in nuclear-associated proteins in the neutropenic mice relative to WT mice. (C) Measurements of BAL nucleic acid concentration at baseline (0 hours), 12 hours, 24 hours, and 48 hours after inducing lung injury show a persistent increase in BAL DNA concentration with a peak occurring 24 hours after injury. (D) Agarose gel electrophoresis of BAL-derived DNA from WT and G-CSF−/− mice 24 hours after injury. (E) Quantification of the distribution of DNA sizes in BAL in the 2 genotypes. (F) Sytox staining of lung through the intratracheal route before fixation. Little Sytox staining is detected in uninjured lungs. Twenty-four hours after injury, however, Sytox staining appears more intense in G-CSF−/− mouse lungs than WT. Scale bar, 250 μm. Each point on the graphs represents 1 animal. The error bars represent the standard error of mean. *P < .05; **P < .01; ***P < .001.
Neutropenic mice have more DNA-containing debris following acid injury of the lung. (A) Lungs from injured areas of WT and G-CSF−/− lungs were fixed 24 hours after unilateral acid-induced lung injury and stained with hematoxylin-and-eosin sections. Scale bar, 100 μm. The extent of debris is apparent. (B) BAL fluid sampled 24 hours after acid-induced lung injury from neutropenic (G-CSF−/−) mice and WT mice were compared using mass spectroscopy after depleting albumin and IgG. We appreciated a marked increase in nuclear-associated proteins in the neutropenic mice relative to WT mice. (C) Measurements of BAL nucleic acid concentration at baseline (0 hours), 12 hours, 24 hours, and 48 hours after inducing lung injury show a persistent increase in BAL DNA concentration with a peak occurring 24 hours after injury. (D) Agarose gel electrophoresis of BAL-derived DNA from WT and G-CSF−/− mice 24 hours after injury. (E) Quantification of the distribution of DNA sizes in BAL in the 2 genotypes. (F) Sytox staining of lung through the intratracheal route before fixation. Little Sytox staining is detected in uninjured lungs. Twenty-four hours after injury, however, Sytox staining appears more intense in G-CSF−/− mouse lungs than WT. Scale bar, 250 μm. Each point on the graphs represents 1 animal. The error bars represent the standard error of mean. *P < .05; **P < .01; ***P < .001.
Neutropenic mice have DNA and DNA-associated proteins in injured lung and BAL fluid
A proteomic analysis of albumin-depleted BAL samples from G-CSF−/− and WT mice was reexamined for repair-associated factors.6 As seen in Figure 1B, BAL of neutropenic mice had increased histones and nucleus-associated proteins (supplemental Table 1). Nucleic acids present in the BAL were increased by 48.5% in G-CSF−/− mice compared with WT (P < .001; Figure 1C). Size-distribution analysis showed a threefold increase (P = .0178) of larger DNA fragments in G-CSF−/− BAL compared with WT (Figure 1D-E). Intra-alveolar Sytox staining was used to quantify extracellular DNA. As seen in Figure 1F, uninjured G-CSF−/− and WT mice did not have increased levels of extracellular DNA. Twenty-four hours postinjury, however, there was a twofold increase (P < .01) in extracellular DNA debris in the G-CSF−/− mice relative to WT.
Neutrophils degrade DNA
To determine whether neutrophils degrade extracellular DNA-containing debris, RNA sequencing was performed in bone marrow (BM)–derived and BAL-isolated neutrophils 24 hours after injury. There was a differential increase in DNA-degrading enzyme gene expression (trex1, dnase1I3, wrn, and isg20) in BAL-derived neutrophils, suggesting that neutrophils acquire DNA-degradation mechanisms following BM release (Figure 2A). Differential increased expression of TREX1 (P = .0002), DNASE1L3 (P = .0045), and WRN (P < .0001) was confirmed by western blot and ELISA on BAL neutrophils compared with BM neutrophils (Figure 2B).
Neutrophils ingest and degrade DNA. (A) Heat map of proteins with DNase activity in neutrophils from BM or BAL. (B) Western blot (TREX1 and DNASE3L1) and ELISA (WRN) analysis of BAL neutrophils compared with BM neutrophils assessing differential protein expression. (C) Bioanalyzer tracings of supernatant after incubation of salmon sperm nuclei either without (top) or with (bottom) BAL neutrophils. The sharp spikes in the tracing without neutrophils represent standards. (D) Ingestion of nuclei by neutrophils. Neutrophils were incubated with Sytox-labeled salmon sperm DNA for 1 hour and neutrophils were stained with anti-Ly6g. Scale bar, 25 μm. Images of neutrophils having taken up DNA are shown. (E) Neutrophils from injured lungs exposed to Sytox through the intratracheal route were recovered by BAL, and stained with anti-Ly6g compared with WT neutrophils. (F) Frames (original magnification ×20) from supplemental Video 1 showing neutrophil engulfment of DNA debris in real time. (G) MyD88−/− neutrophils were impaired in their ability to degrade DNA as assessed by agarose gel. (H) Heatmap of nucleic acid–sensing pattern-recognition receptors in BAL-derived neutrophils compared with BM-derived neutrophils after acid instillation. (I) Histologic section 96 hours after acid injury in a G-CSF−/− mouse, which shows that repair is impaired (left). G-CSF−/− mice were given recombinant Dornase-α (50 μg) via the intranasal route 24 and 48 hours after injury and the degree of repair is markedly improved (right). Scale bar, 100 μm; hematoxylin and eosin stain. Each point on the graphs represents 1 animal. The error bars represent the standard error of mean.
Neutrophils ingest and degrade DNA. (A) Heat map of proteins with DNase activity in neutrophils from BM or BAL. (B) Western blot (TREX1 and DNASE3L1) and ELISA (WRN) analysis of BAL neutrophils compared with BM neutrophils assessing differential protein expression. (C) Bioanalyzer tracings of supernatant after incubation of salmon sperm nuclei either without (top) or with (bottom) BAL neutrophils. The sharp spikes in the tracing without neutrophils represent standards. (D) Ingestion of nuclei by neutrophils. Neutrophils were incubated with Sytox-labeled salmon sperm DNA for 1 hour and neutrophils were stained with anti-Ly6g. Scale bar, 25 μm. Images of neutrophils having taken up DNA are shown. (E) Neutrophils from injured lungs exposed to Sytox through the intratracheal route were recovered by BAL, and stained with anti-Ly6g compared with WT neutrophils. (F) Frames (original magnification ×20) from supplemental Video 1 showing neutrophil engulfment of DNA debris in real time. (G) MyD88−/− neutrophils were impaired in their ability to degrade DNA as assessed by agarose gel. (H) Heatmap of nucleic acid–sensing pattern-recognition receptors in BAL-derived neutrophils compared with BM-derived neutrophils after acid instillation. (I) Histologic section 96 hours after acid injury in a G-CSF−/− mouse, which shows that repair is impaired (left). G-CSF−/− mice were given recombinant Dornase-α (50 μg) via the intranasal route 24 and 48 hours after injury and the degree of repair is markedly improved (right). Scale bar, 100 μm; hematoxylin and eosin stain. Each point on the graphs represents 1 animal. The error bars represent the standard error of mean.
Sytox-labeled salmon sperm nuclei were incubated with BAL-derived neutrophils for 1 hour to determine whether neutrophils directly degrade extracellular DNA. As seen in Figure 2C, no small DNA fragments were detected in samples without neutrophils, but 10-kb fragments were seen in samples incubated with neutrophils. These data suggest that neutrophils degrade extracellular DNA.
Neutrophils engulf extracellular DNA
BAL-derived neutrophils were incubated with or without Sytox-labeled salmon sperm nuclei and stained with Ly6G antibody. Sytox-labeled DNA was identified within neutrophils (Figure 2D) showing the capability to phagocytose extracellular DNA. Analysis by flow cytometry of BAL-derived neutrophils coincubated with Sytox-labeled salmon sperm showed significant increase of neutrophils containing Sytox-labeled DNA compared with control (P = .005; Figure 2E) This was verified by incubating BAL neutrophils with Sytox-labeled DNA using live imaging. Over a 4-hour period, neutrophils were observed binding and phagocytosing Sytox-labeled DNA, offering direct visualization of this process (supplemental Video 1; Figure 2F).
Degradation and engulfment of DNA requires MyD88
To determine how extracellular DNA is degraded, MyD88−/− neutrophils were incubated with Sytox-labeled nuclei. MyD88−/− neutrophils do not engulf nuclei (supplemental Figure 2). Furthermore, MyD88−/− neutrophils failed to degrade extracellular DNA, indicating an MyD88-dependent process (Figure 2G). Expression profiling of BAL neutrophils compared with BM neutrophils during sterile injury (Figure 2H) shows upregulation of Toll-like receptor (TLR) and TLR-associated genes that can sense nucleic acids including: Lrrfip1, Ticam1, and TLR13. Thus, the TLR pathways represent a mechanism through which MyD88 may be necessary for reparative neutrophil function.
Degradation of extracellular DNA is important for lung repair
Conclusion
Inflammation has long been recognized as a double-edged sword, in which tissue damage consequent to neutrophil recruitment is balanced against host defense.18-20 We suggest that beneficial aspects of inflammation extend beyond pathogen clearance, and as others have previously hypothesized, debris disposal is a critical function of recruited neutrophils.21 We recently identified neutrophils as vital for alveolar epithelial regeneration.6 These findings parallel data that neutrophils promote regeneration in various organs22 including liver7 and skin.23 Similar to our data in lung tissue, neutrophils were also important for DNA clearance to promote revascularization in the model of liver injury.7 Our data along with previous studies showing importance of neutrophil-derived matrix metalloproteinase-9 in matrix processing after mechanical lung injury9 implicate the critical role neutrophils have in lung repair and/or regeneration.
We show an important new mechanism of this regenerative pathway by the observation that neutropenia is associated with increased nuclear debris in BAL fluid and in situ. Interestingly, RNA sequencing revealed that alveolar, but not BM, neutrophils express DNA-degradation genes. The kinetics of this neutrophil-acquired phenotype and the location in which it occurs warrant further investigation. Furthermore, alveolar neutrophils incubated with salmon sperm nuclei degrade DNA in an MyD88-dependent manner. Importantly, administration of DNase I proved that DNA-debris degradation improves alveolar repair.
These data elucidate new neutrophil functions during inflammation. Although our data suggest that G-CSF does not mediate macrophage recruitment, macrophages are important in phagocytosis and thus are important for further study of debris clearance and tissue repair. Although the pathologic effects of neutrophils have been described, our data showing neutrophil-dependent DNA-debris clearance and increased alveolar repair highlight benefits of neutrophil responses during inflammation. Of note, DNase I is currently in trials for acute respiratory distress syndrome. The mechanism through which it may improve pathology might in part be clearance of DNA debris. Thus, additional studies are warranted on this unique aspect of neutrophil function in disease pathology.
For original data and reagents, please e-mail the corresponding authors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the following National Institutes of Health grants: 1R01AI099479 from the National Institute of Allergy and Infectious Diseases; 5R01HL10583402, 5T32HL07586, and T32HL715041 from the National Heart, Lung, and Blood Institute; KL2TR001879 from the National Center for Advancing Translational Sciences; and 1F32HL131079-01 from the National Heart, Lung, and Blood Institute.
Authorship
Contribution: J.H.O., A.J.P., and G.S.W. designed the experiments and wrote the paper; A.J.P. performed acid instillation, collected BAL, and fixed lungs; J.H.O., A.J.P., and N.D. collected BAL, and performed cell counts and ELISAs; A.J.P. and G.S.W. did confocal microscopy and image editing; K.G. and M.P. assisted with neutrophil engulfment assays; K.R. assisted with experimental design and neutrophil enrichment/isolation; L.A.S., G.S.W., A.J.P., and S.H.S. generated the proteomic data; and A.J.P. and P.W. performed and analyzed the flow cytometry experiments and data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: G. Scott Worthen, Abramson Research Center, Children’s Hospital of Philadelphia, 416H, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: worthen@email.chop.edu; and Joseph H. Oved, Abramson Research Center, Children's Hospital of Philadelphia 303E, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: ovedj@email.chop.edu.
REFERENCES
Author notes
J.H.O. and A.J.P. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal