In this issue of Blood, Chen et al1 explored the biology underpinning the relationship between expression of ZAP70 and poor disease prognosis in chronic lymphocytic leukemia (CLL). They provide compelling evidence that this tyrosine kinase facilitates CLL progression by promoting malignant cell survival and ability to remodel the microenvironment, and by increasing malignant cell capacity for protein synthesis (see figure). This work thereby lays a solid foundation stone for our understanding of the role ZAP70 plays in CLL.
ZAP70 is a tyrosine kinase that typically functions to mediate proximal T-cell antigen receptor signaling. However, changes in the way the promoter of this gene is methylated in the malignant cells of CLL lead to aberrant expression that correlates with unmutated IGHV gene status and poor disease outcome. Early studies on the function of ZAP70 in CLL cells showed that it enhanced BCR signaling in a way that was independent of its kinase function,2 whereas later studies reported on the relationship between ZAP70 expression and CLL cell migration and ability create a supportive microenvironment.3 The current study ties these observations together with a series of neat biochemical experiments.
Studying the function of ZAP70 in primary CLL cells is problematic because specific inhibitors against this kinase do not exist, and the short life span of primary cells in culture make short interfering RNA-mediated knockdown of proteins with a long half-life difficult. To overcome this problem, the authors’ unique approach is to build on their previous work studying a murine stromal cell line, EL08-1D2, where they show that coculture protects CLL cells from spontaneous apoptosis.4 Although this system stimulates WNT signals in CLL cells, it does not affect their surface expression of immunoglobulin M, which is important because this parameter can change and affect induced signaling when these cells are exposed to cytokine.5 Furthermore, when CLL cells are removed from this coculture system, they regain their susceptibility to spontaneous apoptosis. This leads to the demonstration that ZAP70 expression protects CLL cells from spontaneous apoptosis in the absence of BCR engagement. This is a new finding made more interesting by the authors’ observation that reduction of ZAP70 using short interfering RNA did not overtly affect the strength of induced BCR signaling in their system, an observation that is at odds with a previous study investigating such signaling in ZAP70+ and ZAP70− CLL cells and in CLL cells that ectopically express ZAP70.2 What is intriguing here is that the current manuscript demonstrates ZAP70 association with proteins involved with the signalosome that is formed in CLL cells upon engagement of BCR, raising a question of whether ZAP70 is a nonfunctional bystander in the traditional BCR pathway as we know it. Indeed, this may not be the true function of ZAP70 in this context at all, and the authors provide data showing that induced BCR signaling facilitates interaction of this kinase with ribosomes, where it promotes protein synthesis potentially through association with, and phosphorylation of, ribosome binding proteins such as RPS-17. This is a function specific for ZAP70; the authors show that its paralog, spleen tyrosine kinase, does not associate with these proteins in CLL cells and could also result from weak BCR signals or other stimuli because ZAP70-dependent protein synthesis occurs in CLL cells that are not subject to overt BCR engagement. The cause of such weak BCR signals or stimuli needs further clarification, but could be the consequence of a feedback loop initiated by innate immune signals that the current group previously reported in which secreted immunoglobulin M is able to autorecognize BCR on the surface of ZAP70+ CLL cells.6 Whatever the cause, it is highly probable that this is where ZAP70 plays its most important role, and the data presented show this kinase regulates constitutive activation AKT and gene expression of MYC, CCL3, CCL4, and interleukin 4-induced gene-1 (IL4I1). Thus, weak tonic activation of ZAP70 in CLL cells increases their fitness to survive and proliferate where CCL3, CCL4, and IL4I1 act to recruit T cells and macrophages and help them provide a supportive environment for CLL cells, and where increased levels of MYC in CLL cells drives enhanced proliferation (see figure). How such activation of ZAP70 is connected to unmutated IGHV gene status in CLL now needs to be investigated to determine why expression of this kinase cannot be established as an independent prognostic indicator in this disease.
Now that we more clearly understand the function of ZAP70 expression in CLL, an important question is whether this could be exploited therapeutically. The answer is potentially yes, because cells that have high levels of protein synthesis require essential amino acids to build these proteins. This is supported by studies showing that patients with aggressive CLL have lower levels of serum methionine,7 that CLL cells are acutely sensitive to the absence of cysteine,8 and that higher IL4I1 expression by CLL cells likely increases their catabolism of tryptophan.9 Ultimately, this means that although ZAP70 expression enhances CLL cell fitness, it also exposes them to a need that can be exploited.
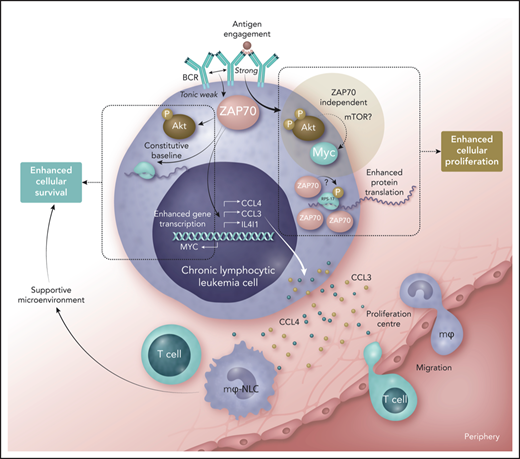
Role of ZAP70 in promoting increased malignant cell fitness in chronic lymphocytic leukemia. Weak tonic signals from the B-cell receptor (BCR), generated by an unclear mechanism, influence ZAP70 to stimulate constitutive activation of AKT, some protein translation, and enhanced transcription of the genes coding for CCL3, CCL4, IL4I1, and MYC. Released CCL3 and CCL4 can stimulate migration of monocytes and T cells into proliferation centers (the former becoming macrophages that serve as nurse-like cells), and these cells provide a supportive microenvironment. In this context, ZAP70 serves to enhance malignant cell survival in CLL. If BCR comes into contact with antigen, strong signals ensue that stimulate association of ZAP70 with ribosomal proteins (such as RPS-17), where the kinase function of ZAP70 may play a role and enhance protein translation. At the same time, the BCR signaling pathway is activated where AKT shows strong activation, leading to increased expression of MYC, but this response is independent of ZAP70. Nevertheless, the outcome of such strong signaling from the BCR is enhanced cellular proliferation. Increased fitness of CLL cells expressing ZAP70 in terms of survival and proliferation are therefore likely to contribute to progressive disease, explaining why expression of this tyrosine kinase is linked with poor prognosis. mTOR, mammalian target of rapamycin; P, phosphate. Professional illustration by Somersault18:24.
Role of ZAP70 in promoting increased malignant cell fitness in chronic lymphocytic leukemia. Weak tonic signals from the B-cell receptor (BCR), generated by an unclear mechanism, influence ZAP70 to stimulate constitutive activation of AKT, some protein translation, and enhanced transcription of the genes coding for CCL3, CCL4, IL4I1, and MYC. Released CCL3 and CCL4 can stimulate migration of monocytes and T cells into proliferation centers (the former becoming macrophages that serve as nurse-like cells), and these cells provide a supportive microenvironment. In this context, ZAP70 serves to enhance malignant cell survival in CLL. If BCR comes into contact with antigen, strong signals ensue that stimulate association of ZAP70 with ribosomal proteins (such as RPS-17), where the kinase function of ZAP70 may play a role and enhance protein translation. At the same time, the BCR signaling pathway is activated where AKT shows strong activation, leading to increased expression of MYC, but this response is independent of ZAP70. Nevertheless, the outcome of such strong signaling from the BCR is enhanced cellular proliferation. Increased fitness of CLL cells expressing ZAP70 in terms of survival and proliferation are therefore likely to contribute to progressive disease, explaining why expression of this tyrosine kinase is linked with poor prognosis. mTOR, mammalian target of rapamycin; P, phosphate. Professional illustration by Somersault18:24.
Conflict-of-interest disclosure: J.R.S. received funding from Verastem Inc. and the Netherlands Translational Research Center B.V., and has a collaborative relationship with BiVictriX Therapeutics. A.J.L. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal