In this issue of Blood, Duval et al painstakingly identified the elusive molecule responsible for the red blood cell Emm antigen, which was first identified in 1973.1 It is, in fact, a free glycosylphoshatidylinositol (GPI) molecule, a glycolipid anchor.
More than 140 human proteins are anchored to the cytoplasmic membrane by a glycolipid composed of glucosamine, mannose, and phosphatidylinositol (ie, GPI). Such proteins include the red blood cell antigens CD55 and CD59, which protect them from hemolysis induced by the complement membrane attack complex. Individuals who have somatic mutations in hematopoietic cells of genes encoding enzymes that are responsible for the synthesis of GPI (such as PIGA and PIGT), do have hemolytic episodes causing paroxysmal nocturnal hemoglobinuria (PNH), treated with a recombinant antibody against the complement protein C5. Individuals with germline mutations in GPI biosynthesis enzymes, however, lack several GPI-anchored proteins throughout their body and are afflicted by multisystem diseases (inherited GPI deficiency disorders) with severe neurologic manifestations such as epilepsy, intellectual disability, and cerebellar atrophy.2 Their neurologic disease is hard to therapeutically address. Supplementation with vitamin B6 to bypass the requirement for membrane-bound alkaline phosphatase and sodium benzoate to increase the transcription of PIGM have been partially helpful in some patients.3-6
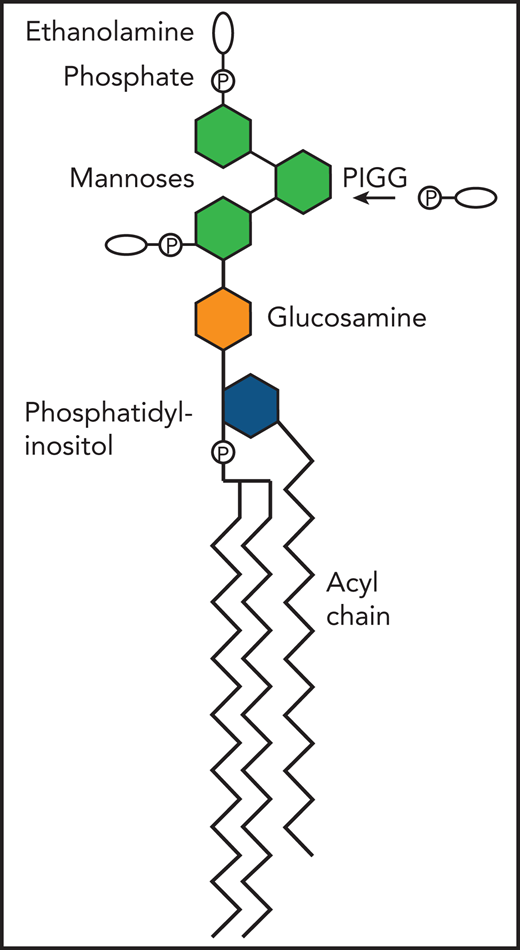
Diagram of free GPI illustrating the addition of ethanolamine phosphate on the second mannose by the PIGG transferase, to thus become the Emm antigen. P, phosphate.
Diagram of free GPI illustrating the addition of ethanolamine phosphate on the second mannose by the PIGG transferase, to thus become the Emm antigen. P, phosphate.
In 1980, at their Montreal congress, the International Society of Blood Transfusion established a working party for blood group terminology.7 As of February 2021, they have identified 43 blood group systems. The Emm system is now system 42, after years of being included in the “901 series” of high-incidence red cell antigens (www.isbtweb.org/working-parties). In this journal, more than 30 years ago, Telen et al8 demonstrated that several high-incidence blood antigens were in fact GPI-anchored proteins, such as the Dombrock antigen (GPI-anchored CD297) and the John-Milton-Hargen antigen (GPI-anchored CD108). These antigens were identified by studying cells from individuals with paroxysmal nocturnal hemoglobinuria. They also noted a decreased response to the Emm antiserum in these cells, thus implicating GPI biosynthesis in the formation of the Emm antigen.
To identify the exact nature of the Emm antigen, Duval et al first sequenced the exome (the coding regions of all genes) of samples from 3 patients described in 1986 who had anti-Emm antibodies. The first of these patients also had neurologic symptoms. They found PIGG mutations in all 3 patients. They confirmed this relationship of the mutations with the absence of the Emm antigen in PIGG knockout cell lines. GPI is in part synthesized by adding ethanolamine phosphate to the second and third mannose of the glycan moiety of GPI (PIGG being responsible for adding it on the second) (see figure). Proteins are then eventually linked to the ethanolamine phosphate, usually the one on the third mannose, via the GPI transamidase complex. The investigators then knocked out PIGS, which is part of the transamidase complex. Not only did they still detect the Emm antigen, they actually noted an increase in it. Collectively, these data pointed to the idea that the Emm antigen was a GPI intermediate synthesized by PIGG, which accumulated on cells when GPI could not be added to proteins. This idea was supported by the fact that the Emm antigen could be detected at a molecular weight of ∼5 kDa on western blots and was thus smaller than GPI-anchored proteins. By knocking out enzymes responsible for different steps in GPI biosynthesis (such as PIGO, which adds ethanolamine phosphate on the third mannose), they deduced that free GPI with ethanolamine phosphate groups on the second and third mannose represented the Emm antigen. Finally, in cells from individuals with certain inherited GPI deficiency (IGDs) (ie, deficiency of PIGO and PIGG), they detected less of the Emm antigen.
The authors mention that the Emm antigen could be used to diagnose IGDs, and although this is technically true, in practice, the diagnosis is made more often through the use of increasingly available broad genomic tests. Flow cytometry can be used as an adjunct to genetic tests, for example, by assessing the cell surface presence of CD16 in granulocytes, an assay sensitive for most IGDs except PIGG deficiency.9 Flow cytometry can be especially useful to tease out variants of unknown clinical significance, or when a mutation can be identified on only 1 of 2 alleles. The Emm antigen may thus be especially useful in these cases to confirm PIGG or PIGO dysfunction when questions remain about the diagnosis after genetic tests.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal