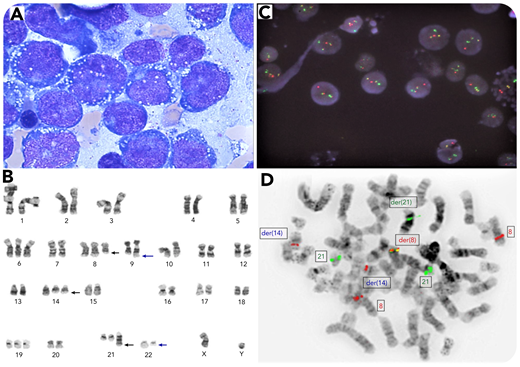
A 75-year-old man with no previous history of hematological disorders presented with 1 week of malaise. Peripheral blood showed leukocytosis with 70% blasts without basophilia or eosinophilia. Bone marrow aspirate revealed 80% blasts with marked vacuolation (panel A; May-Grünwald-Giemsa stain, original magnification ×100). Flow cytometry showed the blasts were CD34+/CD117+/CD13+/CD33+/CD11b+, CD19 dim, cyCD3−/cyCD79a−/CD56−/MPO−. Cytogenetic analysis demonstrated t(9;22)(q34;q11.2), a 3-way t(8;14;21)(q22;q11.2;q22) translocation, and additional numeric abnormalities in all metaphases examined (panel B). Interphase fluorescence in situ hybridization (FISH) was positive for BCR-ABL1 fusion signals in 85% nuclei (panel C; 4′,6-diamidino-2-phenylindole counterstain, original magnification ×1000). Metaphase FISH confirmed the t(8;14;21) and RUNX1-RUNX1T1 fusion on derivative chromosome 8 in all metaphases examined (panel D). Both fusion transcripts were detected by reverse transcription polymerase chain reaction. Next-generation sequencing revealed IDH1 mutation (c.395G>A(p.Arg132His). A diagnosis of acute myeloid leukemia (AML) with BCR-ABL1 and RUNX1-RUNX1T1 was made while noting that blast phase of underlying chronic myeloid leukemia (CML) could not be entirely excluded. The patient was unfit for aggressive chemotherapy and expired after 1 month.
De novo AML with concurrent BCR-ABL1 and RUNX1-RUNX1T1 is rare. BCR-ABL1, a hallmark of CML, occurs in <1% of AML and confers a high-risk disease. AML with RUNX1-RUNX1T1 belongs to the category of core-binding factor (CBF) AML and carries overall favorable prognosis. Although clinical impact is unclear, limited studies suggest that BCR-ABL1 can cooperate with other mutation types as a “class I mutation” and may rarely cooccur in CBF-rearranged AML.
A 75-year-old man with no previous history of hematological disorders presented with 1 week of malaise. Peripheral blood showed leukocytosis with 70% blasts without basophilia or eosinophilia. Bone marrow aspirate revealed 80% blasts with marked vacuolation (panel A; May-Grünwald-Giemsa stain, original magnification ×100). Flow cytometry showed the blasts were CD34+/CD117+/CD13+/CD33+/CD11b+, CD19 dim, cyCD3−/cyCD79a−/CD56−/MPO−. Cytogenetic analysis demonstrated t(9;22)(q34;q11.2), a 3-way t(8;14;21)(q22;q11.2;q22) translocation, and additional numeric abnormalities in all metaphases examined (panel B). Interphase fluorescence in situ hybridization (FISH) was positive for BCR-ABL1 fusion signals in 85% nuclei (panel C; 4′,6-diamidino-2-phenylindole counterstain, original magnification ×1000). Metaphase FISH confirmed the t(8;14;21) and RUNX1-RUNX1T1 fusion on derivative chromosome 8 in all metaphases examined (panel D). Both fusion transcripts were detected by reverse transcription polymerase chain reaction. Next-generation sequencing revealed IDH1 mutation (c.395G>A(p.Arg132His). A diagnosis of acute myeloid leukemia (AML) with BCR-ABL1 and RUNX1-RUNX1T1 was made while noting that blast phase of underlying chronic myeloid leukemia (CML) could not be entirely excluded. The patient was unfit for aggressive chemotherapy and expired after 1 month.
De novo AML with concurrent BCR-ABL1 and RUNX1-RUNX1T1 is rare. BCR-ABL1, a hallmark of CML, occurs in <1% of AML and confers a high-risk disease. AML with RUNX1-RUNX1T1 belongs to the category of core-binding factor (CBF) AML and carries overall favorable prognosis. Although clinical impact is unclear, limited studies suggest that BCR-ABL1 can cooperate with other mutation types as a “class I mutation” and may rarely cooccur in CBF-rearranged AML.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal