In this issue of Blood, Gallego et al1 reveal new complexity in the relationship between the chemokine receptor, CXCR4, and trafficking of dendritic cells (DCs) from the skin to regional lymph nodes (LNs).
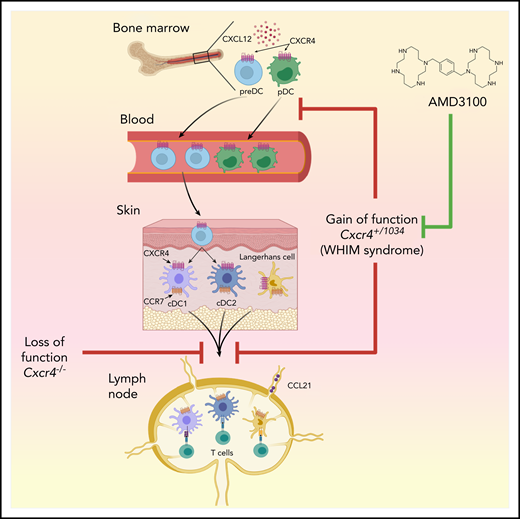
Effect of CXCR4 on leukocyte trafficking. In bone marrow, CXCR4 is expressed on pDCs and preDCs, the latter developing into DC1 and DC2 in peripheral tissues. CXCR4 gain of function in WHIM syndrome, and in Cxcr4+/1013 mice, enhances the interaction of this receptor with its ligand, CXCL12, thereby increasing bone marrow cellularity of multiple leukocyte types, including pDCs and preDCs, and decreasing numbers of these cells in the blood. In the skin, either blockade of CXCR4, or its overactivity, has the same effect of reducing skin DC migration to regional LNs. The CXCR4 inhibitor, AMD3100, reduces skin DC migration in wild-type mice but does not reverse the gain-of-function–dependent reduction in skin DC migration to regional LNs seen in Cxcr4+/1013 mice. Figure generated by Jennifer Martinez, National Institute of Environmental Health Sciences, using Biorender.
Effect of CXCR4 on leukocyte trafficking. In bone marrow, CXCR4 is expressed on pDCs and preDCs, the latter developing into DC1 and DC2 in peripheral tissues. CXCR4 gain of function in WHIM syndrome, and in Cxcr4+/1013 mice, enhances the interaction of this receptor with its ligand, CXCL12, thereby increasing bone marrow cellularity of multiple leukocyte types, including pDCs and preDCs, and decreasing numbers of these cells in the blood. In the skin, either blockade of CXCR4, or its overactivity, has the same effect of reducing skin DC migration to regional LNs. The CXCR4 inhibitor, AMD3100, reduces skin DC migration in wild-type mice but does not reverse the gain-of-function–dependent reduction in skin DC migration to regional LNs seen in Cxcr4+/1013 mice. Figure generated by Jennifer Martinez, National Institute of Environmental Health Sciences, using Biorender.
The importance of CXCR4 to skin immunity in humans was established when heterozygous CXCR4 mutations were found to closely associate with the warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome,2 a rare immunodeficiency associated with high susceptibility to multiple pathogens, including human papillomavirus (HPV). The CXCR4 mutations associated with WHIM syndrome encode carboxyl-terminal domain truncations that prevent desensitization of CXCR4 and are thus gain-of-function mutations. Despite the close association of overactive CXCR4 and WHIM syndrome, however, the underlying pathologic mechanism remains unclear.
To study how CXCR4 desensitization affects leukocyte homeostasis, Gallego et al used a previously described knockin mouse strain that harbors a WHIM syndrome–associated heterozygous mutation in Cxcr4 (Cxcr4+/1013).3 Given that DCs express CXCR4, the authors studied how CXCR4 gain-of-function affects the development and function of plasmacytoid (p)DCs and conventional (c)DCs. They found that in agreement with previous reports, the numbers of circulating pDCs and cDC precursors (preDCs) are reduced in Cxcr4+/1013 mice, probably because these cells are trapped in bone marrow as a consequence of their enhanced interaction of CXCR4 with its only chemokine ligand, CXCL12 (see figure). However, functional analyses of Cxcr4+/1013 pDCs, including their production of interferon-α, did not reveal marked differences between them and their wild-type counterparts. The authors therefore investigated whether Cxcr4+/1013 mice have changes in the number or function of skin DCs. Langerhans cells (LCs) are the only DC population in the epidermis, whereas the dermis contains several DC subsets, including cDC1s and cDC2s. Gallego et al found that, although LCs and dermal DCs were quantitatively preserved in Cxcr4+/1013 mice, the migration of these cells to regional LNs was markedly reduced, both at steady state and in a mouse model of HPV-induced dysplasia. This is a somewhat surprising result, given that a different study had previously shown that the migration of DCs from the skin to regional LNs is dependent on CXCR4.4 Taken together, the 2 studies show that, paradoxically, either blockade or enhanced activity of CXCR4 blocks migration of skin DCs to LNs. In addition, migrated DCs recovered from LNs of Cxcr4+/1013 mice were more activated than their wild-type counterparts, possibly because of the selective egress of only the most activated DCs from skin of Cxcr4+/1013 mice.
It will be important to reconcile how inhibiting or enhancing CXCR4 function has the similar effect of reducing skin DC migration to LNs. CCR7 is well established as the primary chemokine receptor responsible for migration of DCs from peripheral tissue to regional LNs, but Gallego et al found even higher expression of CCR7 in DCs of Cxcr4+/1013 mice than in those of wild-type mice, indicating that CXCR4 must affect DC migration in a way unrelated to CCR7. One possibility is that failure to desensitize CXCR4 leads to retention of DCs in the skin, or the lymphatics, because both tissues produce CXCL12.
Another question is why a reduction in skin DC migration would be associated with increased inflammation? One possibility relates to the immunoregulatory activity of LCs. After migrating to cutaneous LNs, LCs induce anergy, deletion of allergen-specific CD8+ T cells, and activation of Foxp3+ T-regulatory cells.5 Thus, a reduction in LC migration in the Cxcr4+/1013 mice might inhibit immunotolerance and lead to increased inflammation, whereas reduced migration of dermal DCs might inhibit protective immune responses and lead to increased pathogen load. In this regard, it is noteworthy that mutations of other genes that promote DC migration can also lead to enhanced immune responses and autoimmunity. For example, mice lacking CCR7 display increased responses in models of asthma and develop autoimmunity,6 and mice with a DC-specific deletion of Ikkb display impaired DC mobilization from the skin and develop spontaneous autoimmunity,7 although in these 2 mouse strains, the impaired migration likely results from the absence, or insufficient amounts, of CCR7.
The findings by Gallego et al are relevant to ongoing clinical studies. AMD3100, also known as plerixafor, is a selective inhibitor of CXCR4, and is currently US Food and Drug Administration–approved for treatment of non-Hodgkin’s lymphoma and multiple myeloma.8 This drug is also under phase 1 study for WHIM patients (#NCT00967785), and preliminary results indicate that the drug ameliorates multiple aspects of that disease, including panleukopenia, wart burden, and frequency of papillomavirus-associated oropharyngeal squamous cell carcinoma.9 It might be anticipated that AMD3100 treatment of Cxcr4+/1013 mice would reverse their gain-of-function features, including the impaired migration of skin DCs to LNs. However, AMD3100 had little effect on skin DC migration in these animals. AMD3100 did, however, reduce DC migration in wild-type mice, confirming a previous report by Kabashima et al.4 It is unclear why AMD3100 has no effect on skin DC migration in Cxcr4+/1013 mice, but it is possible that WHIM syndrome patients are also resistant to at least some of the effects of AMD3100 on DC migration. Whether that turns out to have a beneficial, or deleterious, effect on adaptive immune responses in these patients remains to be seen.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal